Figure 1.
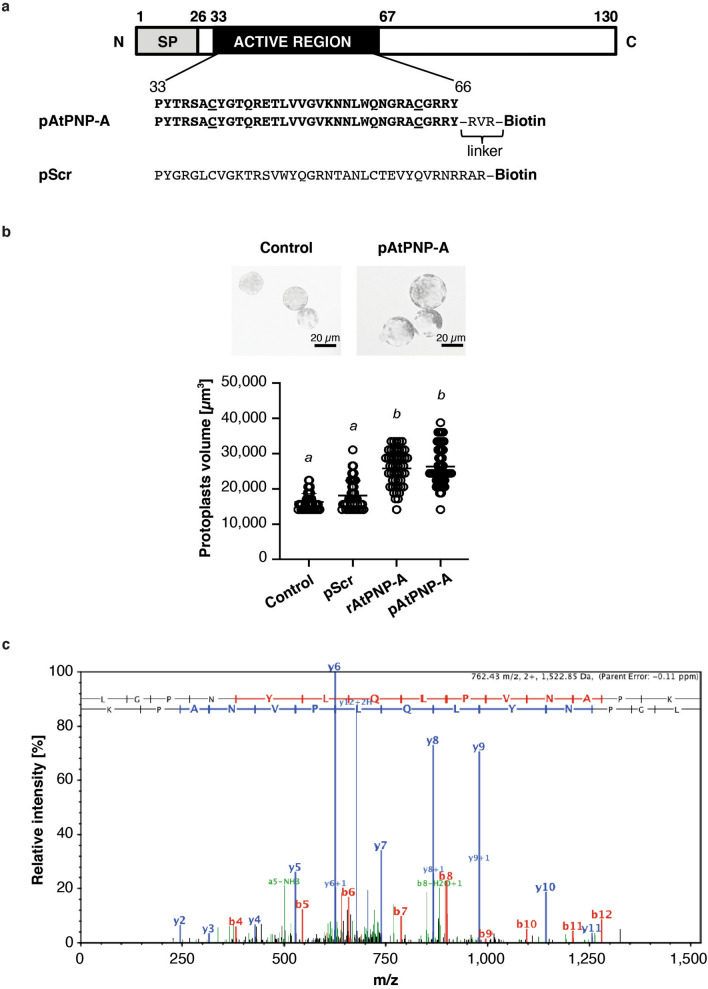
Biologically active peptide containing the active region of AtPNP-A protein binds to CAT2. (a) Domain organization of AtPNP-A and the amino acid sequences of C-terminally biotinylated peptides used in affinity chromatography-based experiments—a peptide containing the active site of AtPNP-A (indicated as pAtPNP-A) or the corresponding scrambled peptide (indicated as pScr). Cysteine residues forming a disulfide bond, characteristic to natriuretic peptide (NP)-like molecules, are underlined. SP, signal peptide. (b) Assaying biological activity of the AtPNP-A peptide (pAtPNP-A) and purified recombinant protein (rAtPNP-A). A. thaliana (Col-0) mesophyll cell protoplasts suspended in 0.4 M mannitol were treated with either water or 100 nM pScr (negative controls) or with 100 nM pAtPNP-A or with 1 μg mL−1 of rAtPNP-A protein for 20 min at room temperature. In each treatment, 50 randomly selected protoplasts with diameter > 20 µm were included in quantitative analysis (scale bar = 20 µm). Protoplast volume was measured and the data obtained from an exemplar experiment are plotted. Columns with different superscript (a and b) indicate significantly different results (mean ± SD, one way ANOVA followed by Tukey–Kramer multiple comparison test, n = 50, P < 0.0001). (c) Exemplar MS/MS spectrum of a unique tryptic peptide of CAT2 (At4g35090) protein.