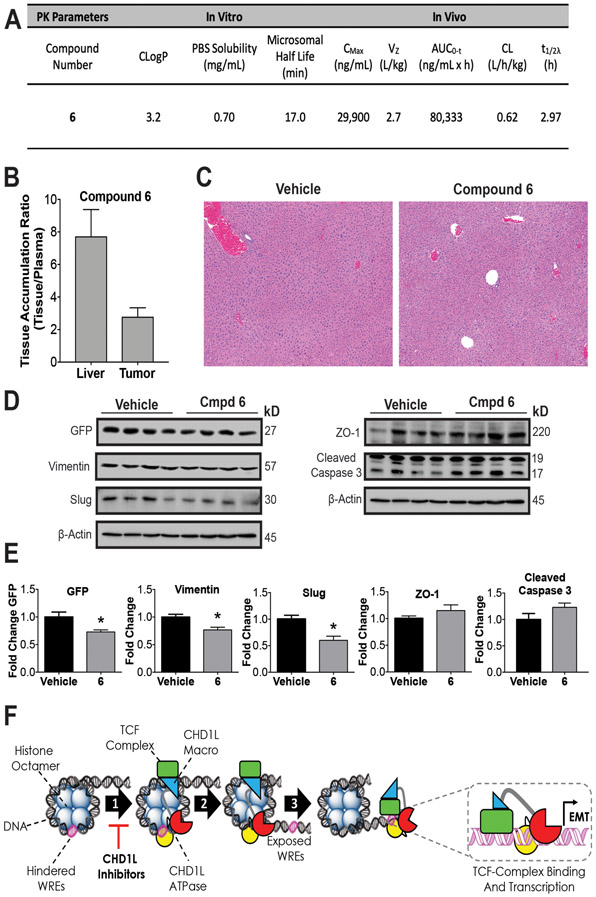
Figure 6: In vivo pharmacology and proposed mechanism of action.
(A) Summary of compound 6 in vivo and in vitro pharmacokinetic parameters. The consensus LogP (CLogP) values were obtained using the SwissADME web tools.(50) Compound 6 was administered by i.p. injection to athymic nude mice QD for 5 days to measure (B) Accumulation in SW620 xenograft tumors and (C) Histopathological assessment of liver toxicity. Representative photomicrograph sections (5x magnification) of liver in both vehicle and compound 6 treated animals. The images demonstrate normal hepatic cord and lobule architecture, with no evidence of hepatocyte degeneration, necrosis, hyperplasia, or parenchymal inflammation. (D) Western blots from xenograft tumors for vehicle and 6 were done to measure PD effects on EMT. (E) Densitometry of blot intensity showing mean value and SEM of four tumor xenograft samples. (F) Proposed mechanism of action of CHD1L mediated TCF-transcription where CHD1L is activated through binding TCF-complex members. (1) Once activated, CHD1L is directed to hindered WREs localized on chromatin. (2) Chromatin remodeling and DNA translocation occurs exposing WRE sites. (3) TCF-complex binds to exposed WREs facilitated by CHD1L, promoting EMT genes and other genes associated with mCRC. CHD1L ATPase inhibitors effectively prevent step 1, leading to the reversion of EMT and other malignant properties of CRC. Welch’s t-test statistical analysis was used to determine significance, where * = P ≤ 0.05