Figure 4.
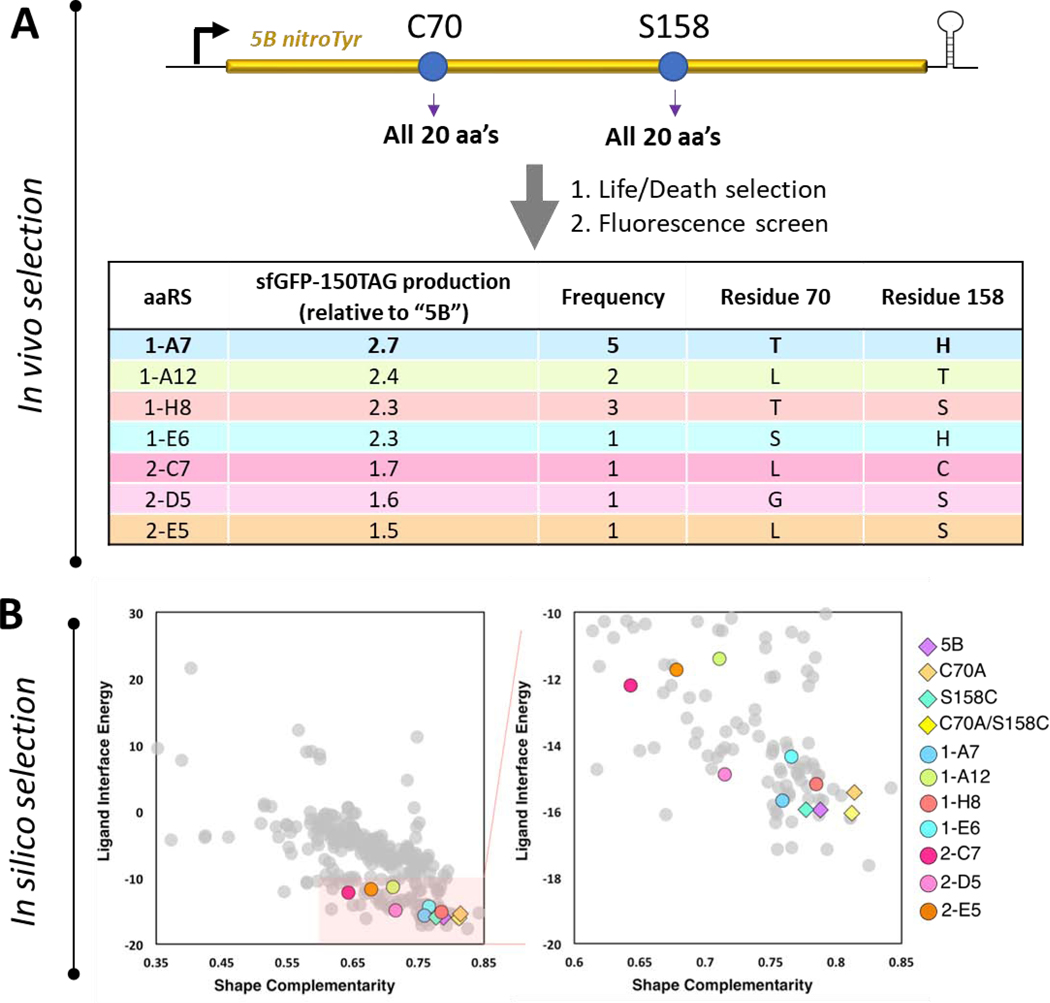
Evaluation of a complete nitroTyr aaRS library at sites 70 and 158 of the “5B” nitroTyr aaRS. (A) The pool of 400 unique protein variants at sites 70 and 158 were selected for their ability to incorporate nitroTyr and no canonical amino acids and surviving members were evaluated for their ability to incorporated nitroTyr into sfGFP-150TAG (see Materials and Methods). Sequences for the top 16 performing hits are indicated, revealing seven unique variants. (B) Rosetta-predicted shape complementarity and ligand interface energy of all 400 variants (left panel), and expanded view of 80 variants with shape complementarity > 0.6 and ligand interface energy < −10 (right panel). The seven new experimental hits (colored circles) and the four previously characterized aaRSs (colored diamonds) were all scored by Rosetta as relatively favorably by these parameters. The top five variants in each category (ligand interface energy and shape complementarity) are listed in Supplemental Table S4.
