Table 2.
Summary of the Most Common Amide Bond Bioisosteres Discussed Herein, Their Physicochemical Properties, and Representative Examples of Pharmacokinetic Effectsa
| Bioisostere | Physicochemical Properties Relative to Amide Bond | Representative Examples1,2 | Design Considerations |
|---|---|---|---|
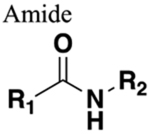 |
Naturally occupies trans configuration. Substituent distance = 3.8–3.9 Å. Dipole moment = approx. 4 Debye. Functions as HBD and HBA. Susceptible to cleavage mediated by proteases, oxidation and hydrolysis (rate of cleavage depends on steric environment). |
||
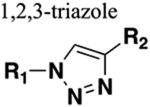 |
Mimics trans configuration upon 1,4-disubstitution (can mimic cis configuration upon 1,5-disubstitution). Substituent distance = 5.0–5.1 Å. Dipole moment = approx. 5 Debye. Functions as HBD and HBA. Resistant to cleavage mediated by proteases, oxidation and hydrolysis. |
Removal of reactive metabolites but substantial decrease in human half-life (compare amide 30 to triazole bioisostere 31). Three-fold increase in metabolic stability (compare amide 20 with triazole bioisostere 23) |
Increased sterics. If carbonyl of amide is a hydrogen bond acceptor the orientation of the nitrogens within the triazole alter distance and angle between donor and acceptor. |
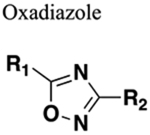 |
Weak base. Functions as HBA only. Electrophilic substitution at carbon difficult. Electrophilic substitution at nitrogen possible with electron-donating group substituents. Resistant to nucleophilic attack but undergo nucleophilic substitution. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. |
Two-three-fold increase in clearance in a rat model (compare amide 43 to oxadiazole bioisostere 45). Reduced metabolic stability (compare amide 47 to oxadiazole bioisosteres 48 and 49). |
Increased sterics. If amine of amide is a hydrogen bond donor this will be lost upon replacement. |
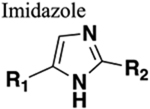 |
Mimics trans configuration. Functions as HBD (if nitrogen unsubstituted) and HBA. Mimics charge of amide nitrogen. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. |
Increased bioavailability (rat) (compare amide 72 to imidazole bioisostere 74). Retained bite angle and N-partial charge (compare amide 81 to imidazole bioisostere 82). |
Increased sterics. If carbonyl of amide accepts two hydrogen bonds one will be lost upon replacement. |
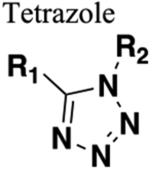 |
Functions as HBA only. Can mimic trans or cis configuration depending on substitution pattern. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. |
102 | Increased sterics. If carbonyl of amide is a hydrogen bond acceptor or the amine a hydrogen bond donor these will be lost upon replacement. |
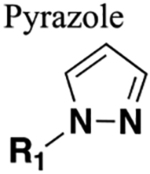 |
Functions as a HBA and HBD (if nitrogen unsubstituted). Distance between substituents greater than in amide. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. |
106 | Increased sterics. Increased lipophilicity. If carbonyl of amide is a hydrogen bond acceptor or the amine a hydrogen bond donor these will be lost upon replacement. |
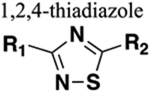 |
Functions as HBA only. Distance between substituents greater than in amide. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. |
111 | Increased sterics. If carbonyl of amide is a hydrogen bond acceptor or the amine a hydrogen bond donor these will be lost upon replacement. |
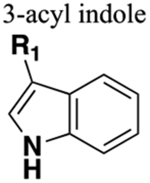 |
Bioisosteric with aryl amide. Much larger. HBD if nitrogen not substituted. Not a HBA as lone pair electrons involved in aromaticity. |
40-fold increase in half-life and seven-fold increase in %F (compare amide 113 with 3-acyl indole bioisostere 114). | Increased sterics. Increased lipophilicity. If carbonyl of amide is a hydrogen bond acceptor this will be lost upon replacement. |
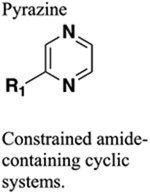 |
Generally only bioisosteric with terminal amides. HBA only. Much larger size. Greater rigidity. Greater rigidity. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. Provide locking of substituents into bioactive configuration. |
120 Increase in target selectivity (compare amide 125 to constrained bioisostere 127). |
Increased sterics. Increased charge. If carbonyl of amide accepts two hydrogen bonds one will be lost upon replacement. Increased sterics. |
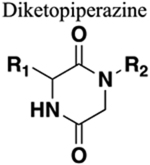 |
Greater rigidity. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. Provide locking of substituents into bioactive configuration. Ability to mimic β-turns. |
134 | Increased sterics. Chain length is increased by a methylene. If amine of amide is a hydrogen bond donor this will be lost upon replacement. Addition of hydrogen bond donor and acceptors maybe advantageous or deleterious to binding. |
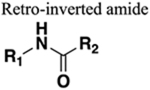 |
Very similar properties to amide but reversed orientation of HBD and HBA. | 139 | No improvement of PK properties. |
 |
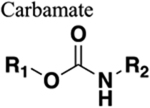
Additional HBA and HBDs. Increases solubility over equally substituted amide, especially when non-symmetrical. Extends chain length by one nitrogen group. |
148 | Increased length by one atom. |
 |
Functions as HBD (if nitrogen unsubstituted) and HBA. Some degree of rigidity. Undergo rapid hydrolysis. |
Exceptional long half-life example 180. Reduced clearance (human microsomes) (compare amide 182 to carbamate bioisostere 183). |
Little to no improvement in PK. |
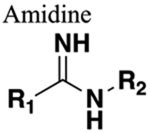 |
One additional HBD (if nitrogen not substituted). Greater charge. Similar distance and rotational constriction. |
Reduced plasma clearance but limited improvement of %F (amidine bioisostere 188). | Increased charged and likely reduction of BBB penetration. |
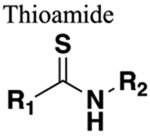 |
Thiocarbonyl bond is longer than carbonyl. C-N bond in thioamide is shorter. Increased rotational constriction. Weak HBA, strong HBD. |
192 | Longer C-S bond may induce steric clash. |
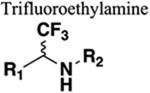 |
Reduced basicity of NH, but can HBD. Similar bond angle (120°). C-CF3 is isopolar with carbonyl group. Increased lipophilicity. Stereochemistry can be manipulated to enhance target binding interactions. |
197 | Steric effects of CF3 compared with O. If carbonyl of amide is a hydrogen bond acceptor this will be diminished or lost entirely upon replacement. |
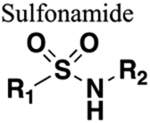 |
Increased water solubility. Additional HBAs. No increase in chain length. |
214 | Increased steric bulk and chain length. Increased water solubility. |
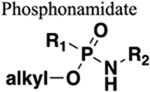 |
Increased water solubility and charge. Stereochemistry may increase (or decrease) interactions with target binding site. Additional HBAs. Metabolically labile. |
233 | Increased charge. Reduced cell permeability. Metabolically labile to different enzymes than amides. |
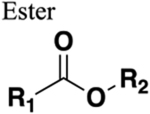 |
Decreased metabolic stability. Can mimic cis or trans conformation. Cannot HBD, weak HBA. |
>Three-fold decrease of half-life (compare amide 239 with ester bioisostere 240). | Deleterious to metabolic stability |
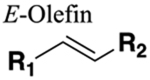 |
Mimic geometry of trans amides. No HBAs or HBDs. Dipole moment much lower (0.1 Debye). Resistant to cleavage mediated by proteases, oxidation and hydrolysis. |
Significantly increased bioavailability (compare amide 241 with E-olefin bioisostere 242). Significantly increased half-life (compare amide 244 with E-olefin bioisostere 245). |
Geometric mimic only. If amide bond is engaged in binding interactions these will be lost upon replacement. |
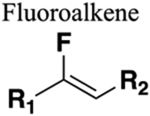 |
(Z)-fluoroalkene mimics the s-trans amide. (E)-fluoroalkene mimics the s-cis amide. Length of C-F bond (1.376 Å) is similar to carbonyl (1.228 Å). C-N and C=C bond length both approx. 1.3 Å. Fluorine has similar van der Waals radius to oxygen. Lower dipole moment (1.4 Debye). Resistant to cleavage mediated by proteases, oxidation and hydrolysis. Increased lipophilicity. |
Significant increase in half-life (>20-fold) in human plasma (compare amide 238 to fluoroalkene bioisostere 275). | Geometric mimic. If carbonyl of amide is a hydrogen-bond acceptor or the amine a hydrogen bond donor these will be lost upon replacement. |
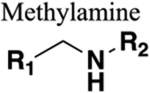 |
Functions as a HBD and acceptor. Free rotation around carbon and nitrogen. Same chain length. |
303 | Increased charge (depending on R2). If carbonyl of amide forms hydrogen bonds these are lost upon replacement. |
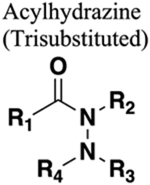 |
Trisubstituted acylhydrazine is bioisosteric to tertiary amide. Similar conformation. Functions as a HBA only. Increased chain length by one nitrogen. Less restricted rotation around N-N bond compared with amide. |
308 | Increased sterics. Increased chain length. If nitrogen of amide is a hydrogen bond donator this will be lost upon replacement. |
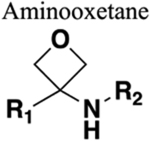 |
Influences basicity of proximal amine. Generally resistant to cleavage mediated by proteases, oxidation and hydrolysis. May increase steric bulk without increasing lipophilicity.294 Decreases planarity. |
Increases metabolic stability. Little details on effect on physicochemical, biological or PIOPD properties. For an overview see reference.295 |
Increased sterics. Loss of trans geometry. |
Footnotes:
Improvements to potency and selectivity are generally excluded as they are most likely due to additional substituent modifications and/or increased target binding and cannot necessarily be attributed to the bioisostere alone.
Comparisons are provided for compounds with the same or very similar substituents to the bioisostere where possible.
