Figure 2. Cross-Reactive mAbs Compete for the Same Antigenic Site and Inhibit Binding to Mxra8 Receptor Protein.
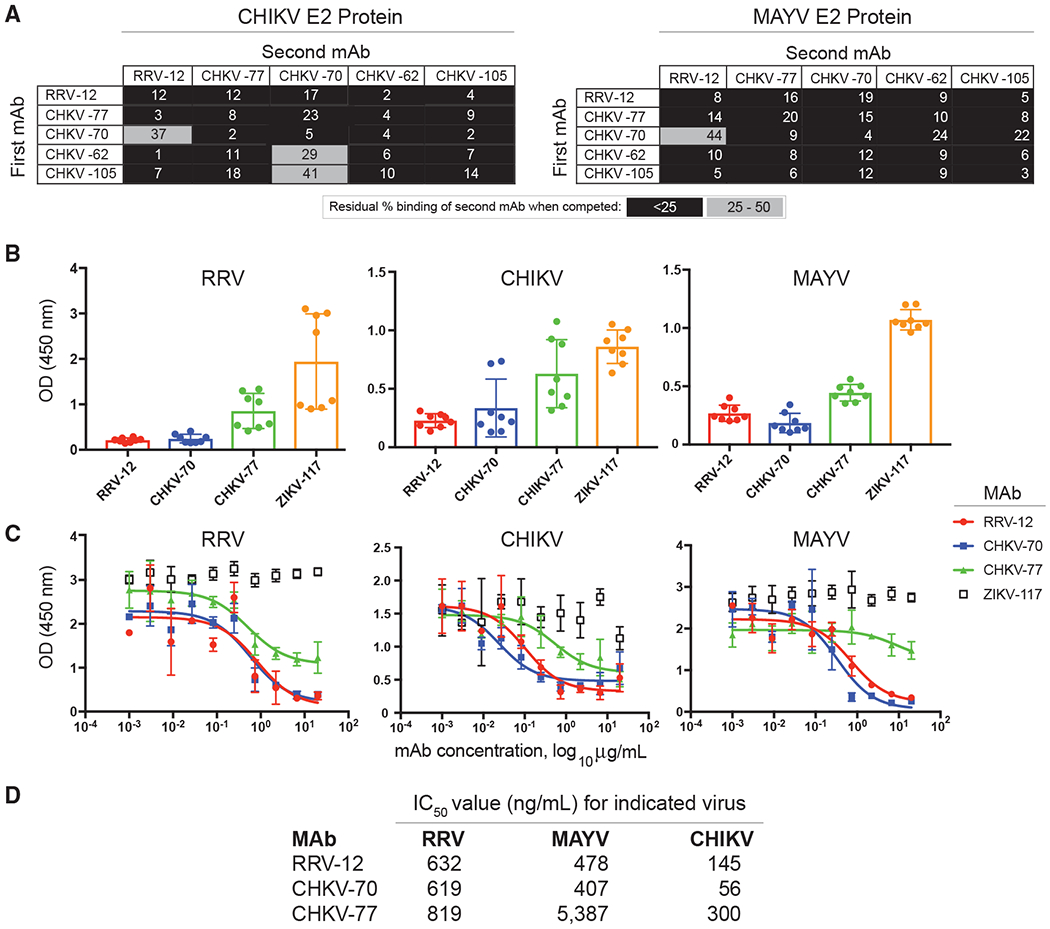
(A) An Octet RED96 instrument (Pall FortéBio) was used to perform epitope-binning studies using competition binding. After a baseline measurement, HIS1K biosensor tips were used to immbolize either CHIKV or MAYV E2 protein containing a histidine tag. After another baseline measurement, biosensors then were transferred to wells containing a first mAb at a concentration of 50 μg/mL for 5 min, before immersion in a solution containing a second mAb, also at a concentration of 50 μg/mL for 5 min. The percent competition of the second mAb in the presence of the first mAb was determined by comparing the maximal signal of binding for the second mAb in the presence of the first antibody to the maximal signal of the second mAb in the absence of competition. Competition was defined by reduction of the maximal binding score to <25% of uncompeted binding (black boxes). A 25% to 50% reduction in maximal binding was considered intermediate competition (gray boxes).
(B) Antibody blocking of virus binding to mouse Mxra8-Fc fusion protein was determined through competition ELISA. MAYV, CHIKV, or RRV were captured on the plate with a human mAb before addition of RRV mAbs followed by Mxra8-mFc (mouse Fc). A loss of signal indicates competition of RRV mAbs with Mxra8-mFc for binding to virus. Two independent experiments were performed in quadruplicate.
(C) Dose-response curve of mAb inhibition of virus binding to mouse Mxra8-Fc fusion protein through competition ELISA. Two independent experiments were performed in triplicate and representative curves are shown.
(D) Half maximal inhibitory values (IC50) values for dose-response curves, calculated based on the average of two independent experiments performed in triplicate.
