Figure 3. RRV-12 Blocks Entry and Cell-Cell Spread of RRV, MAYV, CHIKV, SAGV, GETV, and ONNV.
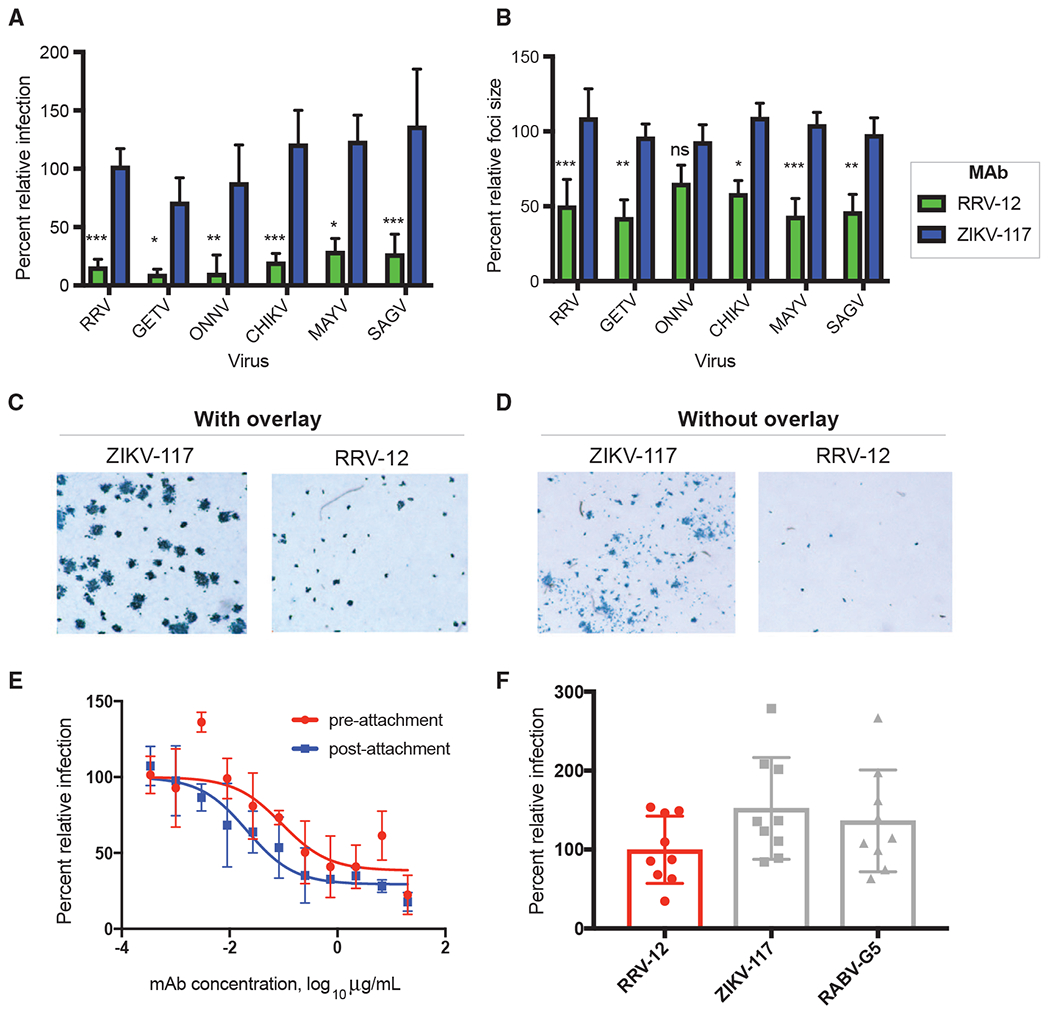
(A) RRV-12 blocks an entry step of RRV, MAYV, CHIKV, SAGV, and GETV. Antibody at a concentration of 20 μg/mL was incubated 1:1 with virus for 1 h at 37°C before addition to Vero cells for 1 h, also at 37°C. Antibody then was removed with 3 washes in medium before addition of a methylcellulose overlay. Cells were incubated at 37°C for 18 h before fixing and immunostaining. Foci were imaged and counted with an automated spot counter. Three independent experiments were performed, with triplicate samples in each experiment. Values were normalized to a virus-only control (One-way ANOVA with Kruskal-Wallis post-test, with mean ± SD compared with ZIKV-117 control; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
(B) RRV-12 blocks cell-to-cell spread of RRV, MAYV, CHIKV, SAGV, GETV, and ONNV as measured through reduction of foci size. Virus was added to Vero cells for 1 h at 37°C before addition of 20 μg/mL of antibody diluted in methylcellulose overlay. After 18 h, cells were fixed and immunostained, and foci size was measured with a CTL Biospot reader. Three independent experiments were performed with triplicate samples in each experiment. Values were normalized to a virus-only control (One-way ANOVA with Kruskal-Wallis post-test, with mean ± SD compared with ZIKV-117 control; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
(C and D) Representative images of RRV-12 reduction of foci size for RRV infection as compared with a control antibody, with antibody added to methylcellulose overlay (C), or with antibody in the absence of methylcellulose overlay (D). Antibody concentrations were at 20 μg/mL, and immunostaining was performed as in FRNT.
(E) Pre-attachment and post-attachment neutralization assay for RRV-12. In the pre-attachment assay, antibody was incubated with virus at 4°C before addition to Vero cells kept at 4°C. For the post-attachment assay, virus was applied to Vero cell monolayer cultures at 4°C before addition of antibody to cells at 4°C. Two independent experiments were performed in triplicate for each antibody, and representative curves are shown.
(F) A FFWO assay was used to measure antibody inhibition of virus fusion with the cell membrane under low-pH conditions. Virus was adsorbed to Vero cell culture monolayers at 4°C for an hour before addition of antibody dilutions, also at 4°C, after removing excess virus. Cells then were exposed to a pH 5.5 medium or a control medium at neutral pH for 2 min and incubated at 37°C. The acidic pH medium was removed, and cells were incubated for an additional 14 h before fixing, permeabilizing, and staining for intracellular virus antigens before flow cytometric analysis. Intracellular virus was quantified by measuring percent PE-positive cells relative to a virus-only control. Three separate experiments were performed in triplicate for each antibody.
