Abstract
Chronic kidney disease is a global healthcare burden, yet clinically-proven treatments are limited. Low-intensity shockwave, which utilizes approximately 10% of the energy levels used in clinically-indicated shockwave lithotripsy, is a promising technique to ameliorate ischemia and regenerate tissues. It has been demonstrated to improve healing in tissues such as bone, muscle, myocardium, and kidney via several mechanisms, particularly through promoting neovascularization. Low-intensity shockwave stimulates mechanoreceptors located primarily in endothelial and proximal tubular cells, and subsequently upregulates vascular endothelial growth factors. This, in turn, promotes angiogenesis and ameliorates renal hypoxia, inflammation, and fibrosis, and ultimately preserves renal function. Furthermore, low-intensity shockwave can stimulate release of homing factors to attract endothelial progenitor or stem cells into injured kidneys for tissue repair. These effects may be beneficial in several kidney disease models, including renal artery stenosis, diabetic kidney disease, and various chronic kidney diseases, although most studies reported to date have been performed in animal models. Due to its low energy intensity, the procedure is relatively tolerable and safe, yet, more clinical studies are needed to establish its efficacy beyond currently-existing strategies. Therefore, low-intensity shockwave therapy emerges as an alternative therapeutic approach that may offer a promising noninvasive intervention for treating renal diseases.
Keywords: low-intensity shockwave, renal artery stenosis, chronic kidney disease, angiogenesis
Introduction
Kidney disease is an important healthcare problem that imposes a significant burden globally, with projected prevalence continuing to rise over the next decade.1 Chronic kidney disease (CKD) shares several risk factors with other cardiovascular diseases and also constitutes an independent risk factor for cardiovascular and non-cardiovascular mortality.2 Strategies to address modifiable risk factors for CKD include optimizing blood pressure and blood glucose control, lipid management, and weight loss. Despite multiple clinical trials, pharmacological therapy to delay CKD remains elusive, with few therapies showing significant clinical benefit. Renin-angiotensin-aldosterone system (RAAS) blockade was the first pharmacological therapy introduced that delayed progression of CKD.3 It took over 2 decades before a new medication was discovered and approved by the FDA, when sodium-glucose cotransporter-2 inhibitors were recently shown to delay progression of diabetic kidney disease (DKD).4 However, medications often require prolonged administration and may pose side-effects precluding their use in some patients. This gap mandates identification of alternative effective options for CKD patients.
Ultrasound shockwave (SW) therapy is a non-invasive modality traditionally used for lithotripsy.5 Because the energy used in SW lithotripsy (SWL) must be sufficiently high to disrupt stones, it may in turn also provoke kidney injury. The degree of SWL-induced injury depends on several factors, including the number, rate, and dose of SWL sessions.6 In order to mitigate these potential adverse effects, low-intensity SW (LiSW) has been adopted. LiSW utilizes only 10% of the energy level in SWL, and has been extensively studied in chronic conditions such as cardiac, musculoskeletal, and genitourinary tract.7–9 These studies have largely shown that LiSW promotes tissue healing by enhancing angiogenesis, mitigating tissue hypoxia, reducing inflammation and fibrosis, and ultimately improving symptoms.7,10 Since many kidney diseases exhibit microvascular loss, ischemia, and inflammation, LiSW has been postulated to potentially improve or even revert these changes in the kidney and ultimately delay CKD progression.
This review aims to present and summarize current evidence regarding the potential of LiSW with a focus on renal conditions. For context, we describe the basic principles underlying LiSW, and its role in non-renal conditions, in which it has been studied more extensively.
What is shockwave?
Shockwave is an acoustic wave, which is defined by an abrupt spike (time between 10% and 90% total initial rise time at the wave front ≤10 nanosecond), high peak-pressure (100MPa), and short life-cycle (10μs).11,12 The instantaneous rise in pressure earned its name of “Shock” wave. It has a low tensile amplitude, broad frequency spectrum (16–20 MHz), and variable negative pressure at its tail.11 SW travels faster than sound (770mph or 1250kph in air)13 and has a definite depth of penetration, exerting several effects along its path.14 This is in contrast to standard ultrasound waves, which consist of periodic oscillations with limited bandwidth.14
SW can be generated by 3 different chief modalities based on electrohydraulic, electromagnetic, or piezoelectric principles.15 Electrohydraulic generators create SW by a spark plug, and SW subsequently propagates in a medium (water) and is eventually focused by a parabolic mirror. Electromagnetic generators, contrarily, generate pressure waves by movement of a magnetic coil, which is then focused by an acoustic lens forming SW. Lastly, piezoelectric generators activate piezoelectric crystals to produce a pressure wave, which is then autofocused to become a SW. The mechanisms underlying each modality and representative machines are shown in Table S1.12,15 Each SW machine has a different maximal energy density ranging from 0.09–1.24mJ/mm2, with frequencies between 1–8Hz (pulse/second) and focal penetration depths between 0–80mm.14 All machines consist of three basic components, including a SW generator, localization system, and positioning system used for focusing on the region of interest.14
Medical application of SW began in the 1980s with SWL for nephrolithiasis.16 Historically, it has been indicated for stones ≤2cm that could not spontaneously pass by conservative management.17 Several factors can affect the success rate of SWL, including stone location, burden, composition, density, and certain patient-related factors.18 The amount of discharge energy used in SWL typically ranges between 12–24kV19 with frequency of 1–1.5Hz (60–90 pulse/min).20 Although initially considered minimally invasive and safe, several animal and human studies suggested that high-energy SW could induce tissue injury in relation to its energy and frequency.6,19 The characteristics of SWL-induced renal injury include focal hemorrhage, small vessel rupture, vascular wall necrosis, podocyte and mesangial cells disruption, ischemic changes in tubular epithelium, and inflammatory cell infiltration. These changes can lead to parenchymal hematoma, proliferative glomerulopathy, nephron loss, interstitial fibrosis, and ultimately CKD.6 Thus, the use of SWL has been declining and replaced by other effective therapies that provide excellent stone-free rates, such as ureteroscopy, which has become the most common modality of definitive stone treatment in several geographical locations.21
Contrarily, LiSW utilizes only 10% of the energy used in SWL, has been shown to induce less tissue injury, and in fact promotes tissue repair in several conditions.7,9,22–24 Given growing interest in this technique, the International Society for Medical Shockwave Treatment (ISMST) has issued a consensus statement on extracorporeal shockwave therapy in numerous conditions (Table S2). Notably, parenchymal kidney disease has not been included in the 2016 published guidelines15, yet emerging LiSW studies in various kidney diseases may change this in the future.
Basic principles of tissue repair by LiSW therapy in non-renal disorders
LiSW exerts its effect by two cardinal mechanisms, which ultimately improve tissue healing by promoting neovascularization and ameliorating inflammatory processes. First, the peak pressure itself renders mechanical stress to tissues and cellular components. Second, LiSW generates cavitation bubbles in the tissues, which later collapse and bestow local effects. These mechanical forces may be converted into cell signaling by upregulation of mechanotransducers, which in turn upregulate proangiogenic factors, including vascular endothelial growth factors (VEGF) and endothelial nitric oxide synthase (eNOS)27, and trans-activate hypoxia-inducible factor-1α.28 LiSW also promotes osteocyte proliferation and enhances bone healing.29 In musculoskeletal disorders, LiSW thereby shows effectiveness in repair of fractures, arthritis and tendinopathies.22,25,26
In cardiac conditions, LiSW has been initially shown to promote angiogenesis and normalize myocardial function in a porcine model7 by upregulating mRNA expressions of VEGF and VEGF-receptor Flk-1, thereby improving capillary densities in the ischemic myocardium.7 Interestingly, LiSW can stimulate heparin sulfate-glycans that act as mechanoreceptors30 and release angiogenic or vasculogenic factors from a reservoir.30 Moreover, LiSW can blunt oxidative stress, reduce inflammation, and facilitate bone marrow-derived stem cells flux into treated area.31–33 LiSW has been subsequently applied clinically, primarily in patients with coronary artery disease and refractory chest pain that failed to resolve despite maximal medical therapy, and its effects were confirmed in placebo-controlled trials and multicenter settings.34,35 However, the long-term effects of LiSW in cardiac conditions remain elusive due to short follow-up periods, and despite its potential benefit, LiSW is currently not an FDA-approved therapy in these patients.
In genitourinary conditions, LiSW has been studied primarily in men with vasculogenic erectile dysfunction (ED).14 LiSW enhances neovascularization in penile and cavernosal vessels, promotes stem cell homing to the penile area36,37, restores α-smooth muscle function, and decreases cavernosal lipid infiltration.38 Meta-analysis of human randomized control trials suggests that LiSW thereby effectively improves ED symptoms.39 Nonetheless, given that the median follow-up in these studies was only 20 weeks, benefits might possibly wane, requiring re-treatment.
Role of SW therapy in renal conditions
Being a highly vascular organ, improving the renal microvasculature and other mechanisms (Figure 1) could plausibly ameliorate kidney pathology and improve outcomes. Indeed, LiSW has been studied in several kidney diseases (Table 1), many of which have shown promising effects.
Figure 1. Mechanisms of SW in repairing kidney injury.
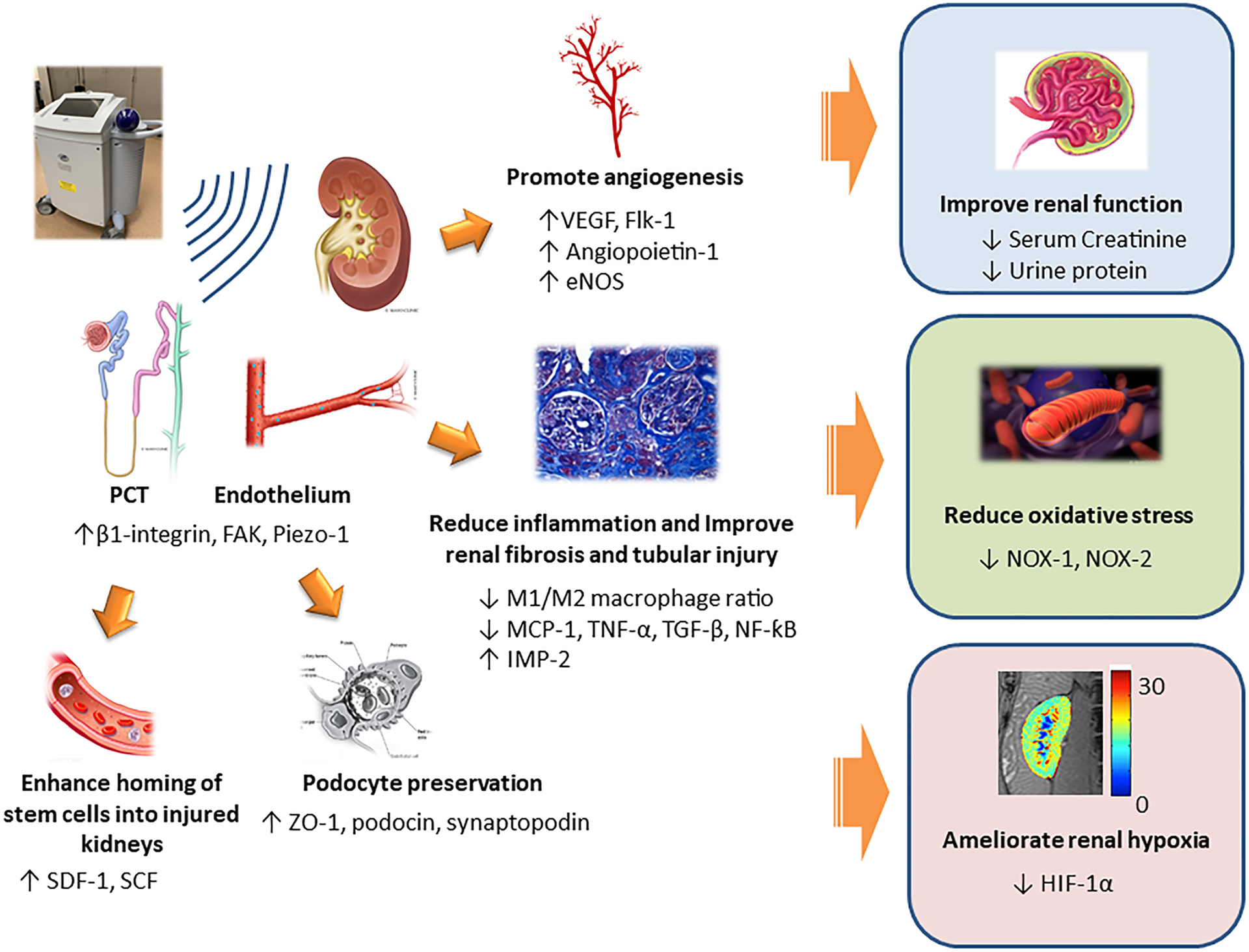
LiSW affects several kidneys cell types, particularly proximal tubules and endothelium, via mechanoreceptors such as β1-integrin, FAK and Piezo-1. Subsequently, angiogenic factors (VEGF, Angiopoietin-1, and eNOS) and receptors (e.g., Flk-1) are upregulated, thus promoting renal angiogenesis. LiSW also suppresses inflammation (macrophages, MCP-1, TNF-α, TGF-β, and NF-ƙB), and increases anti-inflammatory markers (IMP-2), thereby reducing inflammation, tubular injury and fibrosis. The numbers of podocytes are also preserved. Furthermore, LiSW enhances stem cell homing into kidneys by upregulating SDF-1 and SCF. Collectively, these effects translate into improvement in renal function, mitigating oxidative stress, and ameliorating renal hypoxia. eNOS: endothelial nitric oxide synthase, FAK: focal adhesion kinase, Flk-1: VEGF receptor, HIF-1α: hypoxia-inducible factor-1α, IMP-2: integral membrane protein-2, M1: M1 macrophage, M2: M2 macrophage, MCP-1: monocyte chemoattractant-1, NF-ƙB: nuclear factor-ƙB, NOX: nicotinamide adenine dinucleotide phosphate hydrogen oxidase, PCT: proximal convoluted tubule, SCF: stem-cell factor, SDF-1: stromal-derived factor-1, TNF-α: tumor necrosis factor-α, TGF-β: transformation growth factor-β, VEGF: vascular endothelial growth factor, ZO-1; zonula occluden-1
Table 1. Studies of low-intensity shockwave (LiSW) in renal conditions.
| Species | Subjects | Sample size | Interventions | Durations | Effects of LiSW |
|---|---|---|---|---|---|
| Pigs40 | Renal artery stenosis (RAS) swine on atherogenic diet × 6 weeks | 26 |
|
3 weeks |
|
| Pigs41 | RAS swine on atherogenic diet × 6 weeks, followed by revascularization | 26 |
|
3 weeks |
|
| Pigs42 | RAS swine on atherogenic diet × 6 weeks | 24 |
|
3 weeks |
|
| Human44 | Human with diabetic kidney disease (eGFR 30–60 mL/min/1.732) | 14 |
|
3 weeks |
|
| Rats43 | Streptozocin-induced diabetic rats | 30 |
|
6 weeks |
|
| Rats46 | Renal ischemia-reperfusion injury rats model | 37 |
|
16 days |
|
| Rats47 | CKD rat model (5/6 nephrectomy | 40 |
|
2 weeks |
|
Abbreviation list:Ang-1: angiopoietin-1, eNOS: endothelial nitric-oxide synthase, EPC: endothelial progenitor cell, FAK: focal adhesion kinase, Flk-1: VEGF-receptor, eGFR: estimated glomerular filtration rate, HIF-1α: hypoxia-inducible factor-1α, IL: interleukin, IMP-2: integral membrane protein-2, M1: M1 macrophage, M2: M2 macrophage, MCP-1: monocyte chemoattractant-1; MMP-2: matrix metalloproteinase-2, NF-ƙB: nuclear factor-ƙB, NOX: nicotinamide adenine dinucleotide phosphate hydrogen oxidase, PTC: proximal tubular cell, RBF: renal blood flow, SCF: stem-cell factor, SDF-1: stromal-derived factor-1, TNF-α: tumor necrosis factor-α, VEGF: vascular endothelial growth factor.
Renovascular disease
In the first study of LiSW in renal parenchymal disease, we applied LiSW in a porcine model with atherosclerotic renal artery stenotic (ARAS). RAS was induced after 6 weeks of a lipid-rich diet (Table 1), and LiSW (0.09mJ/mm2) administered to the stenotic kidney 3 weeks after RAS induction, bi-weekly for 3 consecutive weeks (total of 6 sessions).40 An ultrasound probe was positioned parallel to the long axis of the stenotic kidney, perpendicular to the SW applicator positioned along the short axis. Then, 200 rapid shots were delivered to each treatment zone throughout the kidney (Figure 2). Four weeks after completion of this regimen, LiSW decreased blood pressure and RAAS activation. Glomerular filtration rate (GFR), renal hypoxia, and blood flow improved in treated ARAS pigs,40 consistent with ameliorated cortical microvascular loss. These proangiogenic effects were supported by upregulation of VEGF and angiopoietin-1 in kidney tissue. Furthermore, LiSW upregulated expression of the mechanotransducers β1-integrin and focal adhesion kinase, primarily in the proximal tubule. This implied that the proximal tubule might be particularly responsive to LiSW compared to other segments of the nephron. No adverse effects were observed in LiSW-treated normal kidneys. Thus, LiSW improved renal structure and function even without revascularization of the stenotic renal artery.40
Figure 2. Low-intensity shockwave application.
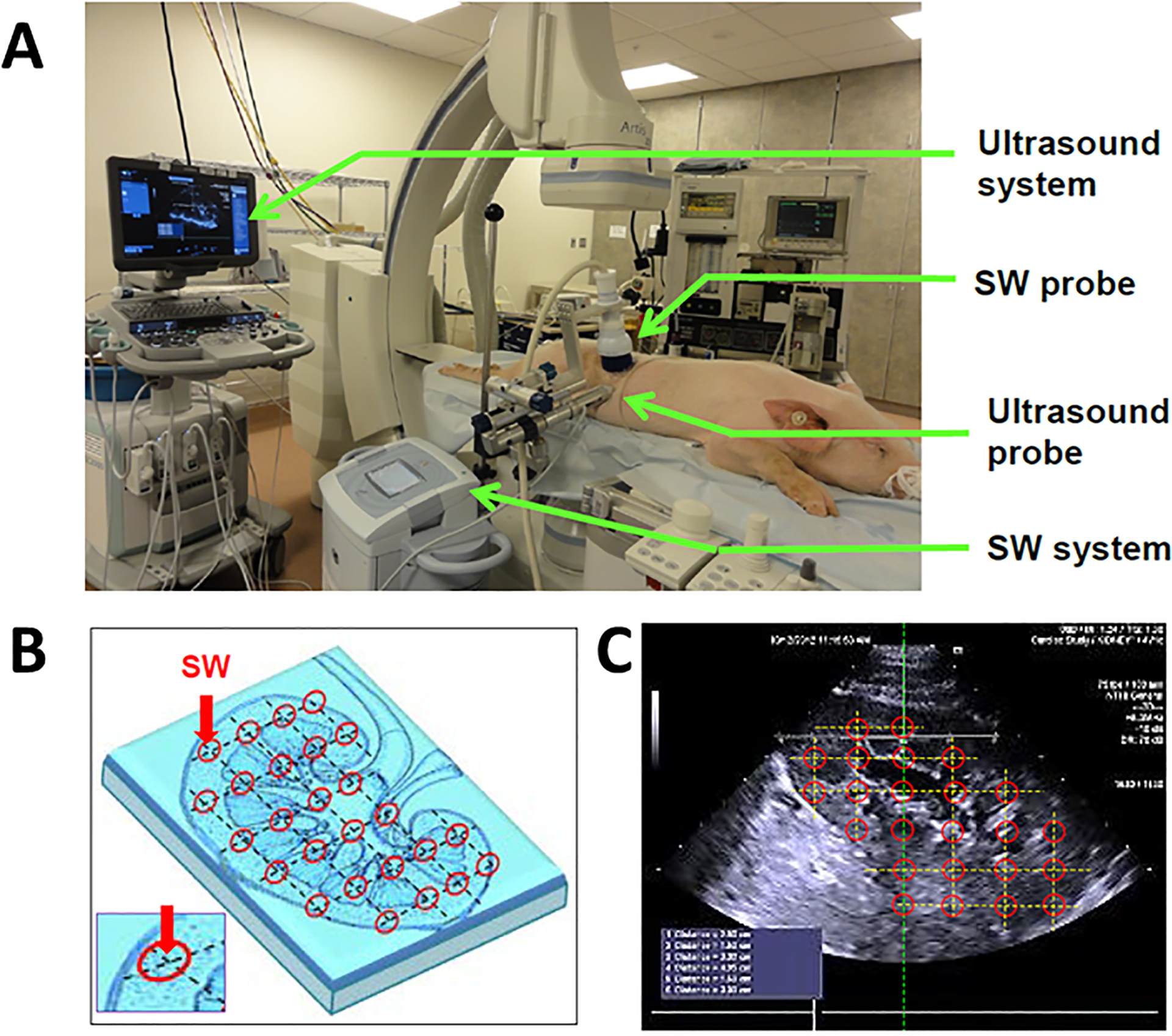
Schematic demonstrating low-intensity shockwave (LiSW) administration in pigs (adapted with permission from Zhang et al40). A: Experimental setting. Green arrows indicate elements in the ultrasound probes, LiSW applicator, and the systems. B: Diagram indicating specific zones of LiSW delivery in the kidney. C: An ultrasound image illustrating LiSW treatment-zones along the short axis of the kidney
The premise of LiSW benefit in RAS kidney was further explored in ARAS pigs undergoing percutaneous transluminal renal angioplasty (PTRA) following completion of a similar LiSW protocol.41 Despite improved blood pressure in PTRA-treated ARAS pigs, GFR remained lower than normal, yet normalized in the group pre-treated with LiSW.41 Similarly, stenotic kidneys in ARAS pigs remained hypoxic after PTRA, whereas LiSW pretreatment permitted improvement in renal oxygenation and a decrease in levels of Inflammatory cytokines.41 Hence, LiSW might precondition the kidney for revascularization.
An additional mechanism by which LiSW might mediate kidney repair involves facilitating homing of reparative endothelial progenitor cells (EPCs) into treated kidneys. In LiSW-treated ARAS pigs, EPC levels were elevated in both the systemic circulation and renal artery compared to untreated ARAS. Moreover, EPCs gradient across treated kidneys were increased, indicating higher retention rate, likely owing to upregulated SDF-1.42
Overall, in a porcine model, LiSW seems to improve post-stenotic kidney function, oxygenation, microvasculature, inflammation and fibrosis by stimulating mechanoreceptors in blood vessels and proximal tubules. Proangiogenic factors are subsequently upregulated, in turn eliciting angiogenesis and ameliorating renal hypoxia. Furthermore, SW can mobilize EPCs and endogenous stem cells into injured kidneys and enhance their reparative capacities. Notably, local delivery of LiSW avoids systemic side effects often observed with systemic interventions like medications. Nevertheless, further study is needed to assess whether LiSW provides additional benefits in subjects with renovascular disease already treated with RAAS blockades.
Diabetic kidney disease
LiSW has also shown promise in animal and human models of DKD. For example, diabetic rats were treated with weekly Li-SW for 6 consecutive weeks (total of 6 sessions) at an energy level of 0.13mJ/mm2 with frequency 200 pulses/min (Table 1). LiSW improved proteinuria, serum creatinine, and fibrosis, enhanced podocyte proliferation, and reduced pro-inflammatory markers (interleukin [IL]-6, IL-1β and M1 macrophages). Again, LiSW was found to upregulate SDF-1 and VEGF.43
In human subjects, a small prospective study aiming to establish the safety of LiSW enrolled 14 patients with DKD (GFR 30–60 ml/min/1.73m2).44 LiSW was applied using Modulith SLX-2 (Table 1) using 4Hz (240 shocks/min) and extended focal size. Each kidney segment (upper, middle and lower) received 1000 shocks (total 3000 shocks/kidney). The energy level used in this study was slightly higher than previously40, initially at 0.136mJ/m2 and gradually increasing to 0.265mJ/m2.44 The protocol included bi-weekly treatments for 3 consecutive weeks (6 sessions), and the patients followed at 1, 3 and 6 months. LiSW stabilized renal function compared to baseline, and tended to reduce albuminuria at 1 and 6 months,44 although these changes have not reached statistical significance. Nonetheless, the safety profile was reassuring, as only 3 patients experienced transient mild macroscopic hematuria. Eleven patients reported mild-to-moderate lower-back tenderness, but this was self-limiting and not associated with other adverse events.44 Another clinical trial (NCT02515461) is currently recruiting patients with moderate DKD is anticipated to be completed by January 2022 (Table S3).
Another exciting application of LiSW in DKD involves tackling the underlying diabetes in order to potentially ameliorate DKD. In rats with streptozotocin-induced diabetes, LiSW improved glycemic control and polyuria.45 LiSW (Evotron) was delivered to the pancreas at 200 shocks once a week for 10 weeks, at energy density of 0.13mJ/mm2 with 200 pulses/min (Table 1).45 LiSW-treated rats had better blood glucose control, possibly due to enhanced pancreatic islets cells and insulin production, which translated into less symptomatic polyuria. LiSW increased β-cells regeneration and decreased inflammatory cytokines including IL-6, TNF-α, and IL-1β. Similar to other organs, LiSW also enhanced angiogenesis by upregulating VEGF and SDF-1.45
Hence, LiSW appears to be safe in human subjects with DKD, and may potentially stabilize renal function in DKD. Moreover, targeting glycemic control by delivering LiSW to the pancreas improves diabetic control, and potentially ultimately renal outcomes. However, additional studies with larger sample sizes are needed to establish the efficacy of this approach in patients with diabetes.
Acute kidney injury
Ischemia-reperfusion (I/R) is an important etiology of acute kidney injury (AKI). LiSW was delivered in I/R mice46 thrice weekly for 3 weeks after I/R (Table 1), 200 shocks at 0.1mJ/mm2.46 LiSW rapidly improved plasma creatinine and decreased tubular injury at 2 days, yet this effect vanished at 20 days. LiSW tended to improve renal fibrosis without reaching statistical significance, probably because the study duration was too short, or perhaps LiSW was initiated too soon after I/R. Interestingly, LiSW preserved lymphatic vessels, which may contribute to the preservation of kidney function after I/R. SW also upregulated mRNA expression of VEGF in the contralateral but not in I/R kidneys.46 Evidently, the underlying etiology of kidney disease determines the response of kidneys to LiSW. While additional studies would be helpful, the early stages of AKI may not constitute an ideal application for LiSW.
Chronic kidney disease
Besides renovascular disease and DKD, a single animal study in CKD applied LiSW to a 5/6 nephrectomy mouse model.47 This study also assessed the effect on kidney function of a combination of LiSW with EPCs and sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor that inhibits SDF-1 degradation and may enhance homing of stem cells into injured kidneys48. LiSW (Storz Duolith) was delivered at 0.12mJ/mm2×180 shocks at days 14, 21 and 28 after CKD (total 3 sessions),47 and kidneys studied at day 60. LiSW improved serum creatinine and urinary protein levels compared to untreated mice, but most effectively in the SW+EPCs+Sitagliptin group. LiSW upregulated SDF-1, systemically increased circulating levels of EPCs47, and diminished fibrosis and inflammation. Podocyte markers were improved by LiSW compared to the EPCs alone, indicating superior podocyte protective effects. Oxidative stress and inflammatory markers were significantly improved in the LiSW group, yet EPC co-treatment was slightly superior to LiSW alone. Moreover, angiogenesis markers (eNOS and CD31) and proangiogenic cytokines were enhanced in all LiSW groups.47
Although studies in CKD remain limited, the results of animal studies appear reassuring. LiSW alone or in combination with cell-based therapy appears to stabilize kidney function, and in fact can promote several reparative mechanisms primarily bestowing pro-angiogenic, anti-inflammatory, and anti-apoptosis benefits. Possibly, adjunctive LiSW might be beneficial when applied in conjunction with additional novel or standard interventions (e.g., RAAS blockade).
Kidney transplant
Although there is currently no report describing LiSW in kidney transplants, the premise of using LiSW in renal allograft is intriguing, especially given the relatively superficial location and ready accessibility of the allograft. Studies are needed to determine whether LiSW may improve allograft outcomes in addition to standard immunosuppression in kidney transplants.
Safety profile of LiSW in parenchymal kidney disease
Historically, due to its high energy, SWL has been linked to renal damage. Various consequent injuries, including intrarenal hemorrhage, ruptured vessels, vascular wall necrosis, and inflammatory cells infiltration, can culminate in chronic changes like interstitial fibrosis and glomerular sclerosis.6 SWL is contraindicated in pregnancy and uncorrected coagulative disorders.49 Bleeding, particularly renal subcapsular hematoma, albeit rare, is a complication of lithotripsy that could adversely affect kidney function, especially in patients with hypertension and obesity50. Microscopic and macroscopic hematuria are common, secondary to parenchymal or vascular injury.51 Interestingly, the corticomedullary junction appears to be the most susceptible area. However, these alterations are often focal and transient.52
Since the energy in LiSW therapy is 1/10th of that used in SWL, far fewer complications and better tolerability are expected. Renal function and urinary protein levels appear to be stable immediately and 4 weeks after LiSW in ARAS pigs, without changes in either blood or urine neutrophil gelatinase-associated lipocalin (NGAL).40 Microscopy revealed no parenchymal hemorrhage or tubular injury immediately after LiSW, with no hematuria observed.40 Contrarily, microscopic hematuria was observed in 21% of DKD patients44, but might have been secondary to the relatively high energy level used in that study, and the rate of hematuria remained lower than post SWL. Furthermore, pretreatment with LiSW can actually prevent renal injury in pigs that receive SWL.53 This suggests that kidney injury from LiSW is minimal and may be reverted by its proangiogenic and anti-inflammatory effects.
Other side effects of LiSW are relatively minor. Many patients generally report a tingling or stinging sensation on the skin during treatment. Pain can occur but is usually mild, transient, and self-limited.44 Subsequent sessions do not aggravate pain and there was no treatment withdrawal due to this complication.44 Interestingly, pain may be related to the degree of parenchymal calcification,44 requiring caution in such patients. No perinephric or subcapsular hematoma has been reported so far in either animal or human studies.40,44 Although LiSW promotes tissue neovascularization, to date development of malignancy secondary to LiSW has not been reported. Nevertheless, application of LiSW should probably be avoided in patients with known malignancy due to theoretical risk of enhancing tumor growth.
Conclusion and future direction
Since instigating the use of extracorporeal LiSW therapy nearly two decades ago, its utility has expanded into numerous medical conditions. Non-invasiveness and ease of application has made LiSW particularly appealing in treating patients at high-risk for invasive procedures. Prior studies using LiSW in musculoskeletal disorders, myocardial ischemia, and ED showed improved outcomes. The chief mechanisms appear to involve upregulation of angiogenic factors, which in turn improve the microvasculature, reduce tissue hypoxia, inflammation and fibrosis, and result in effective tissue healing. Importantly, LiSW upregulates growth and homing factors to mobilize and attract progenitor and stem cells. Animal and human studies have demonstrated safety and often improved outcomes in kidney diseases, including renovascular disease, DKD, and CKD, whereas LiSW may be less effective in AKI. Side effects are usually minor, and include macroscopic hematuria and pain, which are rare and self-limited. Heavy renal calcification may aggravate pain, and these patients should be closely monitored. Additional potentially limiting factors to be considered include machine availability and the need for well-trained technicians. Moreover, because the majority of studies reported in kidney diseases have been performed in animal models, clinical trials in human subjects and other kidney diseases are required to provide a better understanding of LiSW and its clinical utility and efficacy. Importantly, evaluation of the benefits of LiSW on top of standard treatment (e.g., RAAS blockade) is direly needed. Yet, LiSW appears to be a promising novel approach in several kidney diseases, and warrants further exploration.
Supplementary Material
Funding
This study was partly supported by NIH grant numbers: DK120292, DK104273, DK122734, and AG062104.
Disclosures
Dr. Lerman receives grant funding from Novo Nordisk, and is an advisor to AstraZeneca. The authors declare no conflict.
References
- 1.McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD Incidence and Prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30(1):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 3.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334(15):939–945. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 5.Talso M, Tefik T, Mantica G, et al. Extracorporeal shockwave lithotripsy: current knowledge and future perspectives. Minerva Urol Nefrol. 2019;71(4):365–372. [DOI] [PubMed] [Google Scholar]
- 6.McAteer JA, Evan AP. The acute and long-term adverse effects of shock wave lithotripsy. Semin Nephrol. 2008;28(2):200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishida T, Shimokawa H, Oi K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110(19):3055–3061. [DOI] [PubMed] [Google Scholar]
- 8.Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58(2):243–248. [DOI] [PubMed] [Google Scholar]
- 9.Wang CJ, Huang HY, Pai CH. Shock wave-enhanced neovascularization at the tendon-bone junction: an experiment in dogs. J Foot Ankle Surg. 2002;41(1):16–22. [DOI] [PubMed] [Google Scholar]
- 10.Gruenwald I, Appel B, Kitrey ND, Vardi Y. Shockwave treatment of erectile dysfunction. Ther Adv Urol. 2013;5(2):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung B, Preston Wiley J. Extracorporeal Shockwave Therapy. Sports Med. 2002;32(13):851–865. [DOI] [PubMed] [Google Scholar]
- 12.ISMST. Physical principles of ESWT: Basic Physical principles. https://wwwshockwavetherapyorg/about-eswt/physical-principles-of-eswt/.Accessed May 11, 2020.
- 13.Salter CA, Lue TF, Mulhall JP. What Is Shockwave Therapy? The Journal of Sexual Medicine. 2020;17(4):565–569. [DOI] [PubMed] [Google Scholar]
- 14.Chung E, Wang J. A state-of-art review of low intensity extracorporeal shock wave therapy and lithotripter machines for the treatment of erectile dysfunction. Expert Rev Med Devices. 2017;14(12):929–934. [DOI] [PubMed] [Google Scholar]
- 15.ISMST. DIGEST Guideline for Extracorporeal Shock Wave Therapy. https://wwwshockwavetherapyorg/fileadmin/user_upload/ISMST_Guidelinespdf.Accessed May 11, 2020.
- 16.Webb DR, McNicholas TA, Whitfield HN, Wickham JE. Extracorporeal shockwave lithotripsy, endourology and open surgery: the management and follow-up of 200 patients with urinary calculi. Ann R Coll Surg Engl. 1985;67(6):337–340. [PMC free article] [PubMed] [Google Scholar]
- 17.Türk C, Petřík A, Sarica K, et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2016;69(3):475–482. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds LF, Kroczak T, Pace KT. Indications and contraindications for shock wave lithotripsy and how to improve outcomes. Asian J Urol. 2018;5(4):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11(2):310–318. [DOI] [PubMed] [Google Scholar]
- 20.McClain PD, Lange JN, Assimos DG. Optimizing shock wave lithotripsy: a comprehensive review. Reviews in urologyc. 2013;15(2):49–60. [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig WW, Matlaga BR. Urinary Stone Disease: Diagnosis, Medical Therapy, and Surgical Management. Med Clin North Am. 2018;102(2):265–277. [DOI] [PubMed] [Google Scholar]
- 22.Haupt G Use of extracorporeal shock waves in the treatment of pseudarthrosis, tendinopathy and other orthopedic diseases. J Urol. 1997;158(1):4–11. [DOI] [PubMed] [Google Scholar]
- 23.Skolarikos A, Alargof E, Rigas A, Deliveliotis C, Konstantinidis E. Shockwave therapy as first-line treatment for Peyronie’s disease: a prospective study. J Endourol. 2005;19(1):11–14. [DOI] [PubMed] [Google Scholar]
- 24.Uwatoku T, Ito K, Abe K, et al. Extracorporeal cardiac shock wave therapy improves left ventricular remodeling after acute myocardial infarction in pigs. Coron Artery Dis. 2007;18(5):397–404. [DOI] [PubMed] [Google Scholar]
- 25.Schaden W, Fischer A, Sailler A. Extracorporeal shock wave therapy of nonunion or delayed osseous union. Clin Orthop Relat Res. 2001(387):90–94. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J-H, Wang C-J. Biological mechanism of shockwave in bone. International Journal of Surgery. 2015;24:143–146. [DOI] [PubMed] [Google Scholar]
- 27.Wang CJ, Wang FS, Yang KD. Biological effects of extracorporeal shockwave in bone healing: a study in rabbits. Arch Orthop Trauma Surg. 2008;128(8):879–884. [DOI] [PubMed] [Google Scholar]
- 28.Wang FS, Wang CJ, Chen YJ, et al. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem. 2004;279(11):10331–10337. [DOI] [PubMed] [Google Scholar]
- 29.Wang FS, Wang CJ, Sheen-Chen SM, Kuo YR, Chen RF, Yang KD. Superoxide mediates shock wave induction of ERK-dependent osteogenic transcription factor (CBFA1) and mesenchymal cell differentiation toward osteoprogenitors. J Biol Chem. 2002;277(13):10931–10937. [DOI] [PubMed] [Google Scholar]
- 30.Gollmann-Tepekoylu C, Lobenwein D, Theurl M, et al. Shock Wave Therapy Improves Cardiac Function in a Model of Chronic Ischemic Heart Failure: Evidence for a Mechanism Involving VEGF Signaling and the Extracellular Matrix. J Am Heart Assoc. 2018;7(20):e010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu M, Sun CK, Lin YC, et al. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: molecular-cellular and functional assessment. PLoS One. 2011;6(9):e24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Meglio F, Nurzynska D, Castaldo C, et al. Cardiac shock wave therapy: assessment of safety and new insights into mechanisms of tissue regeneration. J Cell Mol Med. 2012;16(4):936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assmus B, Walter DH, Seeger FH, et al. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309(15):1622–1631. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi Y, Ito K, Ito Y, et al. Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J. 2010;74(3):589–591. [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi Y, Ito K, Shindo T, et al. A multicenter trial of extracorporeal cardiac shock wave therapy for refractory angina pectoris: report of the highly advanced medical treatment in Japan. Heart Vessels. 2019;34(1):104–113. [DOI] [PubMed] [Google Scholar]
- 36.Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10(3):738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon SH, Shrestha KR, Kim RY, et al. Combination Therapy Using Human Adipose-derived Stem Cells on the Cavernous Nerve and Low-energy Shockwaves on the Corpus Cavernosum in a Rat Model of Post-prostatectomy Erectile Dysfunction. Urology. 2016;88:226.e221–229. [DOI] [PubMed] [Google Scholar]
- 38.Ruan Y, Zhou J, Kang N, et al. The effect of low-intensity extracorporeal shockwave therapy in an obesity-associated erectile dysfunction rat model. BJU Int. 2018;122(1):133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clavijo RI, Kohn TP, Kohn JR, Ramasamy R. Effects of Low-Intensity Extracorporeal Shockwave Therapy on Erectile Dysfunction: A Systematic Review and Meta-Analysis. J Sex Med. 2017;14(1):27–35. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Krier JD, Amador Carrascal C, et al. Low-Energy Shockwave Therapy Improves Ischemic Kidney Microcirculation. J Am Soc Nephrol. 2016;27(12):3715–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen XJ, Zhang X, Jiang K, et al. Improved renal outcomes after revascularization of the stenotic renal artery in pigs by prior treatment with low-energy extracorporeal shockwave therapy. J Hypertens. 2019;37(10):2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, Santelli A, Zhu XY, et al. Low-Energy Shockwave Treatment Promotes Endothelial Progenitor Cell Homing to the Stenotic Pig Kidney. Cell Transplant. 2020;29:963689720917342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao CC, Huang WH, Cheng KH, Lee CT. Low-Energy Extracorporeal Shock Wave Therapy Ameliorates Kidney Function in Diabetic Nephropathy. Oxid Med Cell Longev. 2019;2019:8259645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skov-Jeppesen SM, Yderstraede KB, Bistrup C, et al. Low-intensity shockwave therapy in the treatment of diabetic nephropathy: a prospective Phase 1 study. Nephrol Dial Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 45.Hsiao CC, Lin CC, Hou YS, Ko JY, Wang CJ. Low-Energy Extracorporeal Shock Wave Ameliorates Streptozotocin Induced Diabetes and Promotes Pancreatic Beta Cells Regeneration in a Rat Model. Int J Mol Sci. 2019;20(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida M, Nakamichi T, Mori T, Ito K, Shimokawa H, Ito S. Low-energy extracorporeal shock wave ameliorates ischemic acute kidney injury in rats. Clin Exp Nephrol. 2019;23(5):597–605. [DOI] [PubMed] [Google Scholar]
- 47.Sung PH, Chen KH, Li YC, Chiang JY, Lee MS, Yip HK. Sitagliptin and shock wave-supported peripheral blood derived endothelial progenitor cell therapy effectively preserves residual renal function in chronic kidney disease in rat-role of dipeptidyl peptidase 4 inhibition. Biomed Pharmacother. 2019;111:1088–1102. [DOI] [PubMed] [Google Scholar]
- 48.Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015;6:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaussy CG, Tiselius HG. How can and should we optimize extracorporeal shockwave lithotripsy? Urolithiasis. 2018;46(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razvi H, Fuller A, Nott L, et al. Risk factors for perinephric hematoma formation after shockwave lithotripsy: a matched case-control analysis. J Endourol. 2012;26(11):1478–1482. [DOI] [PubMed] [Google Scholar]
- 51.Vural A, Oguz Y, Oktenli C, Yenicesu M, Caglar K, Tanboga H. Detection of source of haematuria after extracorporeal shock wave lithotripsy (ESWL) by automated measurement of urinary red cell volume. Int Urol Nephrol. 1998;30(1):31–37. [DOI] [PubMed] [Google Scholar]
- 52.Knapp PM, Kulb TB, Lingeman JE, et al. Extracorporeal Shock Wave Lithotripsy-Induced Perirenal Hematomas. J Urol. 1988;139(4):700–703. [DOI] [PubMed] [Google Scholar]
- 53.Willis LR, Evan AP, Connors BA, Handa RK, Blomgren PM, Lingeman JE. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol. 2006;17(3):663–673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


