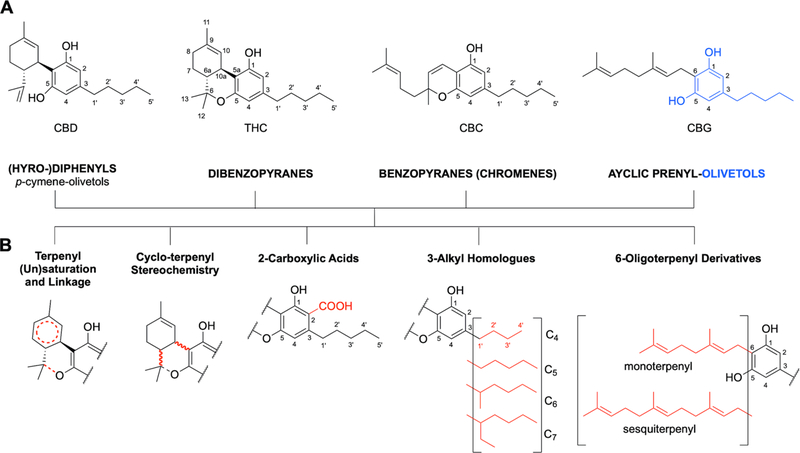
Figure 1.
(A) The tetrahydro-diphenyl skeleton of CBD in the context of the base structures of three other main classes of cannabinoids (dibenzopyranes, benzopyranes, and acyclic prenyl-olivetols); as well as (B) five principal structural variations that occur in all cannabinoid classes. Different combinations of these four classes, with variations such as these presented as well as additional redox-driven metabolic modifications, explain why CBD and THC are just two molecules within the complex metabolome of cannabinoids.