Figure 5. Activation of NPYDBH sympathetic cells switched to promote inflammation following LPS pre-exposure.
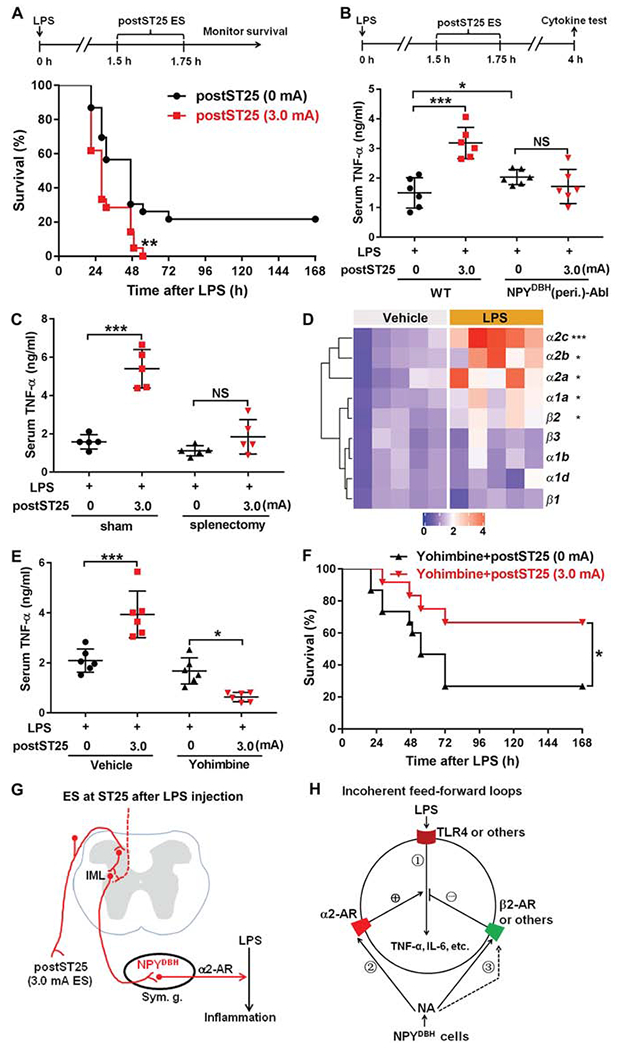
(A) 3.0 mA postST25 ES, performed 1.5 hours after LPS exposure, increased lethality in LPS-treated C57BL/6J mice (log-rank test; 0 mA, n = 23; 3.0 mA, n = 22; **P = 0.006).
(B) Loss of 3.0 mA postST25 ES-evoked increase in serum TNF-α in NPYDBH (peri.)-Abl mice compared with wild type (WT) mice (F1, 20 = 5.602, P < 0.05; ***P < 0.001; NS, P = 0.271).
(C) Splenectomy, compared with sham surgery, blocked postST25 ES-evoked increase in serum TNF-α (F1, 16 = 39.871, P < 0.001; ***P < 0.001; NS, P = 0.125).
(D) Heat map of quantitative RT-PCR results, showing changes in various adrenergic receptor (AR) expression 1 hour after LPS injection (two-side student’s unpaired t-test; β1: t8 = −0.049, P = 0.962; β2: t8 = −3.840, **P = 0.015; β3: t8 = −0.671, P = 0.521; α1a: t8 = −3.764, **P = 0.016; α1b: t8 = −0.876, P = 0.406; a1d: t8 = −0.270, P = 0.794; α2a: t8 = −2.957, *P = 0.018; α2c: t8 = −6.328, ***P < 0.001; α2b: Mann-Whitney Rank Sum test, *P = 0.016).
(E) α2-AR antagonist Yohimbine blocked postST25 ES-evoked increase of TNF-α (F1, 20 = 59.709, P < 0.001;***P < 0.001, *P = 0.016).
(F) Co-treatment with Yohimbine allowed 3.0 mA postST25 ES to promote survival for LPS-treated C57BL/6J mice (log-rank test, *P = 0.045).
(G) postST25 ES drive NPYDBH cell-dependent pro-inflammatory pathways, via activation of α2 ARs. (H) Schematic description of incoherent feed-forward loops. ES-evoked noradrenaline release from NPYDBH cells modulates LPS-induced TNF-α production (via activation of TLR4) in splenic cells, via activation of β2 and a2 ARs that produce the negative and positive modulatory legs, respectively.
n = 22–24 mice (A, F) and 5–6 mice for all other groups. NS: not significant. Data are shown as mean ± SEM.
