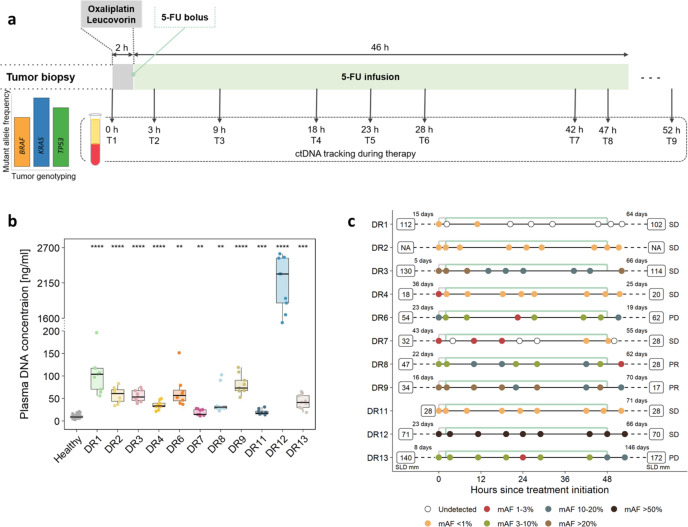
Fig. 1. Study outline and plasma parameters.
a Schematic overview of the FOLFOX regimen and the time points of blood collection. All tumor tissues were analyzed for mutations in KRAS, and in some cases, BRAF and TP53 were sequenced as well. b Plasma DNA quantities of the patients and healthy controls (n = 60) (Student’s t-test; **p < 0.01, ***p < 0.001, ****p < 0.0001). All boxplots indicate the minimum and maximum value and median (center), and the interquartile range is shown by box and whiskers. c The median mutant allele frequencies (mAFs) for each plasma DNA analysis and the timing of each blood draw are displayed. Furthermore, the sum of longest diameters (SLD) as established by CT imaging for the selected target lesions according to RECIST are shown prior to (left side) and after (right side) completion of the FOLFOX cycle.