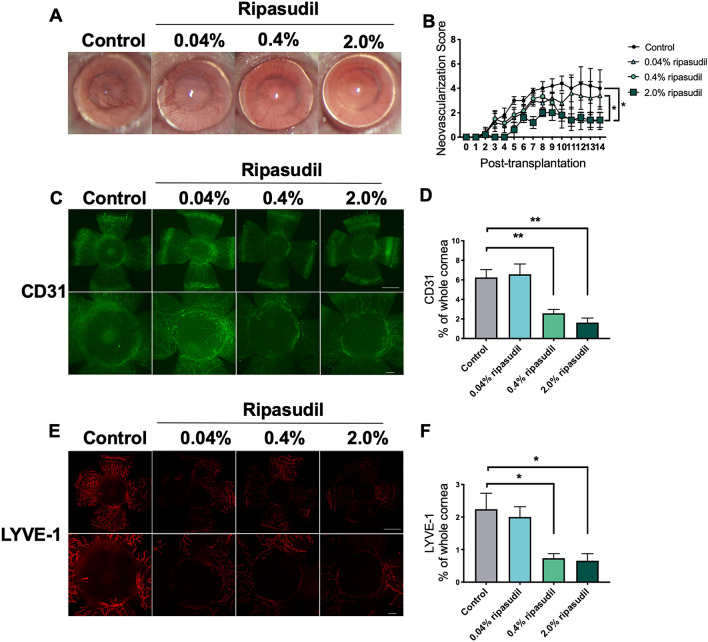
Figure 1.
Topical administration of ripasudil suppressed neovascularization and lymphangiogenesis in murine corneal transplants. (A) Representative slit-lamp images showing grafted corneas on day 14 post-transplantation (magnification × 25). (B) Neovascularization scores of grafted corneas 14 days after transplantation (two-way ANOVA, n = 5 per group; *p < 0.01). (C) Representative immunohistochemical images of CD31 staining of grafted corneas on day 14 post-transplantation. Upper row: whole grafted cornea. Bottom row: high magnification showing center of grafted cornea. (D) Percentages of the corneal area covered with blood vessels (CD31+) in the ripasudil and control groups (one-way ANOVA, n = 5 per group; *p < 0.01). (E) Representative immunohistochemical images of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) staining of grafted corneas on day 14 post-transplantation. Upper row: whole grafted cornea. Bottom row: high magnification showing center of grafted cornea. (F) Percentages of the corneal area covered with lymphatic vessels (LYVE-1+) in the ripasudil and control groups (one-way ANOVA, n = 5 per group; *p < 0.05). The area of the whole grafted cornea covered by blood or lymphatic vessels was analyzed using ImageJ version 1.53a (National Institutes of Health, Bethesda, MD, USA; available at https://rsb.info.nih.gov/ij/index.html)57,58. Scale bar, 1000 μm. ANOVA analysis of variance.