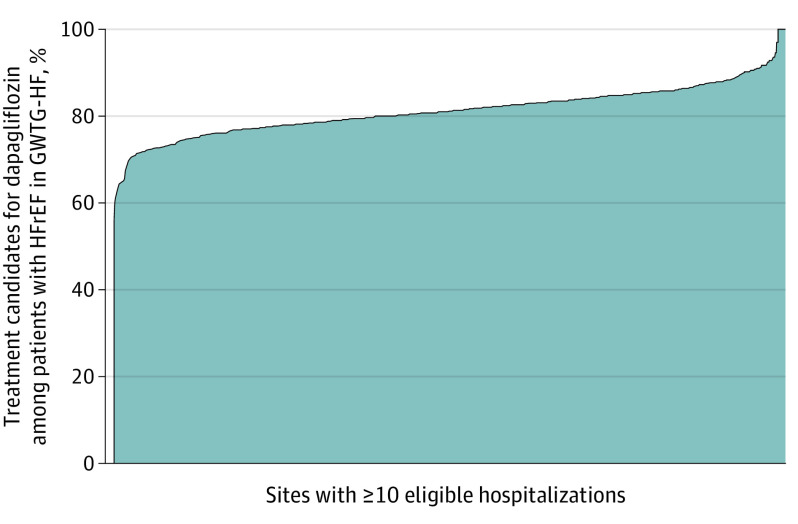
Figure 2. Proportion of Patients With Heart Failure With Reduced Ejection Fraction (HFrEF) at Each Get With the Guidelines–Heart Failure (GWTG-HF) Site Meeting US Food and Drug Administration (FDA) Label Criteria for Dapagliflozin.
Ordered histogram displaying the proportion of patients meeting FDA label criteria for dapagliflozin of all eligible patients with HFrEF at each participating site in GWTG-HF. We included only hospitals with at least 10 eligible hospitalizations for HFrEF during the study period (355 sites, 154 522 patients). There was a median of 81.1% treatment candidates per site (interquartile range, 77.8%-84.6%) (range, 56.0%-100%). The x-axis indicates the distribution of sites (ordered from lowest to highest).