FIGURE 2.
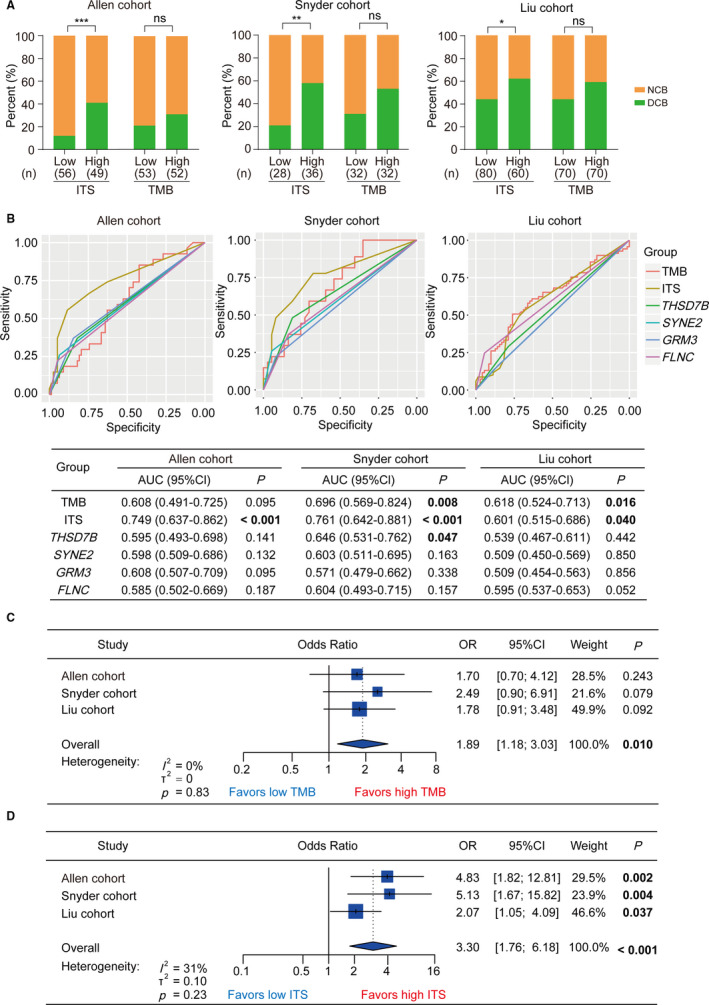
The prediction of durable clinical benefit from ICIs therapy by ITS and TMB. (A) Clinical benefit from ICIs therapy stratified by ITS and TMB in the Allen cohort, Snyder cohort and Liu cohort. (B) ROC curve analysis for prediction of durable clinical benefit from ICIs therapy by ITS, TMB, THSD7B, SYNE2, GRM3, and FLNC in the Allen cohort, Snyder cohort, and Liu cohort, respectively. P value was calculated by the comparison between tested AUC and reference AUC (equal to 0.5). (C) Forest plot showing univariate logistic regression and meta‐analysis for durable clinical benefit, taking TMB as the input variable in the Allen cohort, Snyder cohort, and Liu cohort. (D) Forest plot showing univariate logistic regression and meta‐analysis for durable clinical benefit, taking ITS as the input variable in the Allen cohort, Snyder cohort, and Liu cohort. ICIs, immune checkpoint inhibitors; ITS, immunotherapy score; TMB, tumor mutation burden; DCB, durable clinical benefit; NCB, no clinical benefit; ROC, receiver operator characteristic; AUC, area under curve; CI, confidence interval; OR, odds ratio; ***p < .001, **p < .01, *p < .05; ns, no significance
