Abstract
Objective
Severe or critical patients with coronavirus disease 2019 (COVID-19) are at increased risk for developing acute kidney injury (AKI). However, the rate of AKI in patients of different severities and independent predictive factors associated with AKI are not well understood.
Patients and Methods
We enrolled 107 severely or critically ill elderly patients with COVID-19 who were admitted to the intensive care unit (ICU) in Wuhan, China. AKI was defined according to the 2012 KDIGO criteria. We explored the association between AKI and in-hospital mortality using logistic regression. A predictive nomogram was formulated to predict the AKI development of patients with COVID-19 based on multivariate logistic regression.
Results
A total of 107 elderly patients were enrolled during the study period. The mean age was 70 (64–78) years, and 69 (64.5%) were men. For the 107 patients, the degree of severity of COVID-19 was categorized as 37 patients with the severe type (34.6%) and 70 patients with the critical type (65.4%). Overall, 48 of the 107 patients (44.9%) developed AKI during their hospitalization, while AKI occurred in 7 (18.9%) out of the 37 severe patients and 41 (44.9%) out of the 70 critical patients. Of the AKI patients, 35.4% (17/48) required continuous renal replacement therapy, including 14.3% of AKI patients in severe cases and 39.0% of AKI patients in critical cases. Kaplan–Meier analysis demonstrated that patients with AKI had a significantly higher risk for in-hospital mortality than severely and critically ill patients without AKI. Multivariate logistic regression analysis showed that AKI (OR = 33.74; 95% CI = 3.34–341.29; P = 0.003), septic shock (OR = 15.58; 95% CI = 2.08–116.78; P = 0.008), invasive mechanical ventilation (OR = 18.44; 95% CI = 2.35–144.69; P = 0.006), and oxygenation index (OR = 0.99; 95% CI = 0.98–1.000; P = 0.014) were independent risk factors for in-hospital mortality. A nomogram was established based on the multivariate analysis results. The C-index for the developed AKI model was 0.935 (95% CI, 0.892–0.978); when 10-fold cross validation was used to validate the model, the corrected C-index was 0.825.
Conclusion
AKI is common among COVID-19 patients admitted to the ICU and is recognized as a marker of disease severity. The proposed nomogram accurately predicted AKI development in ICU patients with COVID-19 based on individual characteristics. Therefore, the strategy for kidney protection against severe or critical pneumonia is appropriate.
Keywords: coronavirus disease 2019, acute kidney injury, diagnosis, nomogram, in-hospital mortality
Background
A novel coronavirus has caused coronavirus disease 2019 (COVID-19), which was first discovered in December 2019 in Wuhan, China, and has rapidly become an international outbreak of respiratory illness.1,2 Similar to severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), COVID-19 is rapidly evolving and expanding, resulting in many hospitalizations, serious complications, admission to the intensive care unit (ICU) and death.3–6 Despite significant improvements in health-care technology in the ICU, such as the use of mechanical ventilation (MV), renal replacement therapy (RRT), and extracorporeal membrane oxygenation (ECMO), the mortality of severe COVID-19 patients remains unacceptably elevated.7 One reason for this phenomenon may be that much attention was focused on pulmonary complications, while little attention was paid to other organ dysfunctions, such as acute kidney injury (AKI), which initially was reported with low incidence and could be considered negligible.6,8
In fact, AKI is a severe complication of COVID-19, especially in severe patients. The actual incidence in the ICU remains uncertain and may have been underestimated due to either the retrospective design of the studies or the lack of clear AKI definitions.7,9,10 The close relationship between AKI and coronavirus infection was previously identified in the SARS and MERS epidemics, with an incidence of AKI in SARS of 6.7%, whereas the AKI prevalence was as high as 43.0% in MERS.11 According to data from Europe and the United States, AKI is prevalent in hospitalized patients with COVID-19 and has been reported to occur in 37% to 46% of patients overall and up to 68% of patients admitted to the ICU.12,13 Furthermore, patients with serious infections, comorbid disease, and predisposing factors (eg, MV, sepsis, hypovolemia, and nephrotoxic drug use) were associated with AKI occurrence, and mortality was high in these AKI patients (SARS, 91.7%; MERS, 70.0%).14,15 It is therefore important for clinical management to understand whether accompanying AKI is a bystander phenomenon or an important marker of disease severity and a negative prognostic factor for survival.
Early recognition of AKI in COVID-19 and intensive surveillance and therapeutic measures to limit subsequent AKI or progression to more severe stages are crucial to reduce morbidity and mortality. Therefore, we aimed to investigate the incidence and diagnosis of AKI upon admission to the ICU in COVID-19 patients, examine the potential association between AKI and in-hospital mortality with different severities, and generate a nomogram model to predict AKI.
Patients and Methods
Study Design and Patients
We retrospectively analyzed patients diagnosed with COVID-19 hospitalized from February 4, 2020, to April 16, 2020. All patients who were enrolled in this study were diagnosed with COVID-19 according to the guidance provided by the Chinese National Health Commission. This study was approved by the National Health Commission of China and the Institutional Review Board at Huo Shen Shan Hospital (HSSLL028, Wuhan, China). The requirement for written informed consent was waived by the ethics committee of the designated hospital for patients with emerging infectious diseases. We excluded patients younger than 18 years, those who had chronic kidney disease (CKD) stage 4–5, those who had less than 2 serum creatinine (Scr) examinations, and those who had a missing or incomplete medical history. Patients who only had 2 Scr assays with intervals longer than 7 days were also excluded because we could not ensure whether AKI developed.
Data Collection
After careful medical chart review, epidemiological history, clinical information, laboratory test, radiological assessment, complication, diagnosis during the hospital course, treatment and outcome data were obtained with data collection forms from electronic medical records and reviewed by a trained team of nephrologists. The information recorded included demographic information, medical history, exposure history, underlying comorbidities, symptoms and signs, laboratory findings, chest computed tomographic scans, clinical management (ie, antiviral therapy, corticosteroid therapy, intravenous immunoglobulin therapy, respiratory support, continuous renal replacement therapy [CRRT], and ECMO), and outcomes (including length of stay, discharge, and mortality). All patients' data accessed complied with relevant data protection and privacy regulations.
Definitions
The Scr criteria in the KDIGO guidelines were used for screening because retrospectively collected urine data can be inaccurate. The baseline Scr level was defined as the most recent measurement in the previous 3 months.16 When there were no prior Scr records, we used the lowest Scr value during hospitalization as the baseline Scr level.17,18 The peak Scr level was the highest Scr level reached during the episode. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition.19 Septic shock was defined according to the Sepsis-3 criteria.20 Severe COVID-19 was defined if at least one of the following items was satisfied:21 (a) breathing rate ≥30/min; (b) pulse oximeter oxygen saturation (SpO2) ≤93% at rest; or (c) ratio of partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ≤300 mmHg (1 mmHg=0.133 kPa). Critical illness was defined if at least one of the following items was satisfied: (a) respiratory failure occurred, and the individual required MV; (b) shock; or (c) failure of other organs, and the individual received care in the ICU.
Statistical Analysis
Continuous parametric variables are presented as the means ± standard deviations (SDs) or medians with interquartile ranges based on the distribution of the variables. Categorical variables are presented as numbers (n) or percentages (%). Between-group comparisons of continuous variables were performed using Student’s t-test or the Mann–Whitney U-test, and Pearson’s chi-squared or Fisher’s exact test was used for categorical variables. The probability of survival was estimated using the Kaplan–Meier method, and curves were compared using the Log rank test. Univariate and multivariate logistic regression analyses were performed to study the relationship between AKI and mortality; variables with statistical significance were introduced in the stepwise logistic regression to adjust the effects of other covariates on the relationship between AKI and mortality. Furthermore, we developed a prediction model for AKI occurrence based on the variables at ICU admission. We used lasso logistic regression to select risk factors. Risk factors were selected when the Akaike Information Criterion (AIC) of the model reached a maximum. We included these selected variables in the final logistic regression to establish the prediction model for AKI. A nomogram was presented based on the prediction model we developed. Finally, the model was validated using 10-fold cross validation. The Harrell concordance index (C-index) was used to assess the performance of the prediction model. The higher the C-index is, the more accurate the model is. Statistical analyses were performed using SAS-9.3 and R version 4.0.2 to conduct the analysis. P-values <0.05 were considered to indicate statistical significance.
Results
Study Population
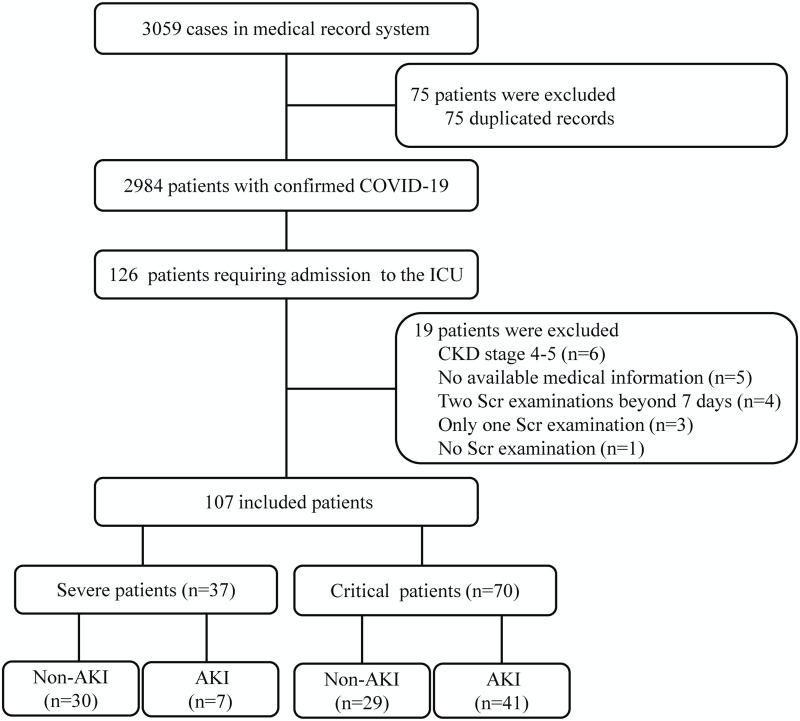
A total of 3059 consecutive hospitalized patients with confirmed COVID-19 were enrolled in this study between February 4 and April 16, 2020, and 126 of them were severe- or critical-type patients that required admission to the ICU. We ultimately excluded 19 patients, resulting in 107 elderly patients who were eligible for the final analyses (Figure 1).
Figure 1.
Flowchart of the patient inclusion and exclusion process.
The baseline characteristics of patients at ICU admission are provided in Table 1. The median age was 70 years; 64.5% of them were male (69/107), and 35.5% were female (38/107). The median duration from onset of symptoms to hospital admission was 15 (10–24) days and to ICU admission was 21 (14–32) days. The median length of hospital stay was 21 (10–36) days, and the length of ICU stay was 9 (4–15) days. Hypertension (73, 68.2%), cardiovascular disease (33, 30.8%), chronic obstructive pulmonary disease (23, 21.5%), diabetes (22, 20.6%), and cerebrovascular disease (19, 17.8%) were the most common comorbidities. One hundred patients (93.5%) showed multiple mottling and ground-glass opacities in the lungs on initial radiographs, and 106 patients (99.1%) showed bilateral patchy shadowing. Of the 107 patients, there were 37 patients with the severe type (34.6%) and 70 with the critical type (65.4%). Ninety-nine patients (92.5%) required oxygen therapy, 67 (62.6%) were treated with noninvasive MV, and 55 (51.4%) were treated with invasive MV. CRRT was used in 20 (18.7%) patients, and ECMO was used in 4 (3.7%) patients, none of whom survived. The most frequently observed complications included ARDS in 48 (44.9%) patients, AKI in 48 (44.9%), hypoproteinemia in 41 (38.3%), septic shock in 38 (35.5%), and disseminated intravascular coagulation in 9 (8.4%) during hospitalization.
Table 1.
Comparisons of the Clinical Characteristics of Patients in ICU with Different Severities of Coronavirus Disease 2019
| Characteristics | All Patients (n=107) | P-value | Severe Patients | P-value | Critical Patients | P-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-AKI (59, 55.1) | AKI (48, 44.9) | Non-AKI (30, 81.1) | AKI (7, 18.9) | Non-AKI (29, 55.1) | AKI (41, 44.9) | ||||
| Age (years) | 68 (63–75) | 73 (67–81) | 0.031 | 67 (63–78) | 78 (71–84) | 0.021 | 68 (63–72) | 72 (65–81) | 0.170 |
| Male sex | 37 (62.7) | 32 (66.7) | 0.671 | 18 (60.0) | 6 (85.7) | 0.174 | 19 (65.5) | 26 (63.4) | 0.856 |
| Body mass index (kg/m2) | 22.8±1.9 | 23.7±2.5 | 0.033 | 22.7±1.9 | 22.7±2.5 | 0.930 | 23.0±1.9 | 23.9±2.5 | 0.092 |
| Comorbidity | |||||||||
| Hypertension | 37 (62.7) | 36 (75.0) | 0.175 | 20 (66.7) | 6 (85.7) | 0.294 | 17 (58.6) | 30 (73.2) | 0.202 |
| Cardiovascular disease | 12 (20.3) | 21 (43.8) | 0.009 | 6 (20.0) | 4 (57.1) | 0.058 | 6 (20.7) | 17 (41.5) | 0.068 |
| Chronic obstructive pulmonary disease | 12 (20.3) | 11 (22.9) | 0.747 | 5 (16.7) | 0 | 0.132 | 7 (24.1) | 11 (26.8) | 0.800 |
| Diabetes | 10 (16.9) | 12 (25.0) | 0.305 | 3 (10.0) | 1 (14.3) | 0.750 | 7 (24.1) | 11 (26.8) | 0.800 |
| Cerebrovascular disease | 6 (10.2) | 13 (27.1) | 0.023 | 2 (6.7) | 3 (42.9) | 0.025 | 4 (13.8) | 10 (24.4) | 0.275 |
| Chronic kidney disease | 0 | 5 (10.4) | 0.004 | 0 | 3 (42.9) | 0.001 | 0 | 2 (4.9) | 0.140 |
| Signs and symptoms | |||||||||
| Cough | 51 (86.4) | 38 (79.2) | 0.317 | 26 (86.7) | 5 (71.4) | 0.353 | 25 (86.2) | 33 (80.5) | 0.528 |
| Fever | 46 (78.0) | 35 (72.9) | 0.545 | 24 (80.0) | 4 (57.1) | 0.225 | 22 (75.9) | 31 (75.6) | 0.981 |
| Dyspnoea | 47 (79.7) | 30 (62.5) | 0.049 | 47 (79.7) | 30 (62.5) | 0.049 | 24 (82.8) | 27 (65.9) | 0.117 |
| Muscle ache | 38 (64.4) | 30 (62.5) | 0.838 | 16 (53.3) | 2 (28.6) | 0.231 | 22 (75.9) | 28 (68.3) | 0.490 |
| Fatigue | 34 (57.6) | 27 (56.3) | 0.886 | 18 (60.0) | 3 (42.9) | 0.412 | 16 (55.2) | 24 (58.5) | 0.779 |
| Chest pain | 11 (18.6) | 5 (10.4) | 0.235 | 7 (23.3) | 0 | 0.069 | 4 (13.8) | 5 (12.2) | 0.844 |
| Chills | 11 (18.6) | 5 (10.4) | 0.235 | 5 (16.7) | 0 | 0.132 | 6 (20.7) | 5 (12.2) | 0.340 |
| Headache | 4 (6.8) | 2 (4.2) | 0.554 | 3 (10.0) | 0 | 0.251 | 1 (3.4) | 2 (4.9) | 0.769 |
| Diarrhoea | 1 (1.7) | 3 (6.3) | 0.212 | 0 | 0 | – | 1 (3.4) | 3 (7.3) | 0.479 |
| Chest CT findings | |||||||||
| Multiple mottling and ground glass opacity | 56 (94.9) | 44 (91.7) | 0.500 | 28 (93.3) | 7 (100.0) | 0.352 | 28 (96.6) | 37 (90.2) | 0.292 |
| Pneumonia | 0.274 | 0.514 | – | ||||||
| Unilateral pneumonia | 1 (1.7) | 0 | 1 (3.3) | 0 | 0 | 0 | |||
| Bilateral pneumonia | 58 (98.3) | 48 (100.0) | 29 (96.7) | 7 (100.0) | 29 (100.0) | 41 (100.0) | |||
| MAP on ICU admission | 97±15 | 95±21 | 0.633 | 101±14 | 95±11 | 0.268 | 92±15 | 95±23 | 0.495 |
| Laboratory results on ICU admission | |||||||||
| Albumin (g/L) | 31.9±3.8 | 31.0±4.5 | 0.265 | 31.9±3.3 | 33.4±5.7 | 0.559 | 31.6±4.3 | 30.5±4.2 | 0.294 |
| Alanine aminotransferase (U/L) | 30.5 (16.4–57.7) | 31.4 (17.2–53.3) | 0.698 | 22.6 (12.0–53.9) | 21.7 (15.2–30.2) | 0.628 | 35.9 (18.4–62.8) | 36.0 (17.3–59.3) | 0.981 |
| Aspartate aminotransferase (U/L) | 26.9 (16.9–42.0) | 29.7 (21.9–63.7) | 0.152 | 24.3 (14.9–39.9) | 26.5 (19.7–53.9) | 0.698 | 31.9 (19.1–48.7) | 30.2 (22.6–73.1) | 0.551 |
| BUN (mmol/L) | 6.1 (4.5–8.1) | 9.4 (6.2–13.4) | <0.001 | 5.4 (4.4–6.7) | 9.1 (4.1–12.0) | 0.103 | 6.7 (4.7–9.0) | 9.5 (6.4–13.6) | 0.007 |
| Uric acid (μmol/L) | 189.0 (133.0–229.0) | 282.5 (187.5–392.3) | <0.001 | 190.5 (146.5–228.0) | 315.0 (229.0–502.0) | 0.002 | 170.0 (116.5–235.0) | 261.0 (181.5–387.5) | 0.001 |
| Creatine kinase (U/L) | 44.5 (25.1–85.8) | 66.3 (36.4–160.6) | 0.025 | 32.1 (19.8–72.3) | 66.7 (53.1–75.8) | 0.092 | 52.1 (36.9–101.3) | 63.7 (35.9–164.1) | 0.489 |
| Lactate dehydrogenase (U/L) | 305.2 (225.0–375.3) | 467.0 (293.1–755.2) | <0.001 | 228.3 (198.8–306.2) | 251.0 (242.0–578.3) | 0.194 | 354.9 (303.1–469.3) | 482.1 (334.0–773.9) | 0.031 |
| Creatine kinase isoenzyme (U/L) | 11.9 (8.7–16.1) | 18.1 (11.1–30.2) | <0.001 | 9.3 (7.9–11.9) | 11.0 (8.8–52.7) | 0.181 | 15.7 (11.4–21.5) | 18.6 (12.6–29.8) | 0.116 |
| Cystatin C (mg/L) | 1.0 (0.9–1.2) | 1.4 (1.1–1.8) | <0.001 | 1.0 (0.9–1.2) | 1.6 (1.3–2.2) | <0.001 | 1.1 (0.9–1.3) | 1.3 (1.1–1.7) | 0.011 |
| CO2 (mmol/L) | 25.0 (23.0–28.0) | 22.0 (19.0–25.0) | 0.001 | 24.0 (23.0–27.0) | 23.0 (20.0–29.0) | 0.656 | 26.0 (22.0–29.0) | 22.0 (19.0–24.0) | 0.002 |
| D-dimer (mg/L) | 1.8 (1.3–5.4) | 5.1 (2.4–7.9) | <0.001 | 1.7 (0.9–3.2) | 4.5 (2.4–8.0) | 0.052 | 2.4 (1.5–6.2) | 5.4 (2.3–9.1) | 0.028 |
| Haemoglobin (g/L) | 114±20 | 119±20 | 0.224 | 110±19 | 116±20 | 0.451 | 118±21 | 120±21 | 0.816 |
| Leucocytes (×109/L) | 9.0 (6.2–13.2) | 10.7 (6.1–17.4) | 0.261 | 7.1 (5.6–10.4) | 5.0 (4.2–5.6) | 0.015 | 11.2 (7.2–15.7) | 11.7 (8.1–18.2) | 0.633 |
| Platelets (×109/L) | 224 (165–276) | 153 (60–196) | <0.001 | 224 (169–260) | 163 (79–166) | 0.008 | 224 (101–298) | 152 (58–198) | 0.005 |
| C-reactive protein (mg/L) | 33.2 (8.7–112.4) | 91.5 (22.5–150.9) | 0.031 | 22.4 (5.7–86.0) | 22.1 (2.0–117.5) | 0.816 | 52.4 (11.8–141.2) | 94.1 (28.6–155.4) | 0.226 |
| Procalcitonin (ng/mL) | 0.1 (0.1–0.4) | 0.5 (0.2–1.4) | <0.001 | 0.1 (0.1–0.2) | 0.2 (0.1–3.2) | 0.087 | 0.2 (0.1–0.6) | 0.6 (0.2–1.4) | 0.022 |
| Blood gas analysis | |||||||||
| Lactate (mmol/L) | 1.2 (0.7–2.3) | 2.1 (1.3–3.7) | 0.001 | 1.0 (0.7–1.3) | 1.2 (1.1–1.8) | 0.206 | 1.8 (1.2–2.7) | 2.2 (1.5–3.7) | 0.170 |
| PH | 7.42±0.10 | 7.39±0.12 | 0.110 | 7.43±0.05 | 7.41±0.03 | 0.192 | 7.41±0.13 | 7.38±0.13 | 0.402 |
| PaO2 (mmHg) | 78.9 (56.7–114.0) | 71.3 (57.0–90.4) | 0.503 | 88.9 (74.7–123.4) | 81.8 (78.0–121.5) | 0.877 | 59.9 (53.7–83.5) | 70.7 (57.0–84.6) | 0.238 |
| PaCO2 (mmHg) | 38.7 (33.4–45.2) | 39.9 (32.9–46.7) | 0.558 | 37.8 (33.4–40.6) | 37.6 (32.0–47.5) | 0.641 | 41.9 (33.1–48.0) | 40.7 (33.2–46.7) | 0.802 |
| Oxygenation index (mmHg) | 205.0 (86.0–280.0) | 91.0 (64.0–179.0) | 0.003 | 254.5 (203.0–324.0) | 233.0 (86.0–281.0) | 0.313 | 86.0 (65.0–203.0) | 87.0 (61.0–154.0) | 0.616 |
| Proteinuria | 13 (22.0) | 33 (68.8) | <0.001 | 5 (16.7) | 4 (57.1) | 0.035 | 8 (27.6) | 29 (70.7) | <0.001 |
| Hematuria | 19 (32.2) | 28 (58.3) | 0.007 | 6 (20.0) | 3 (42.9) | 0.225 | 13 (44.8) | 25 (61.0) | 0.182 |
| Kidney function | |||||||||
| Baseline Scr (μmol/L) | 58.0 (48.0–66.0) | 72.0 (62.0–78.0) | <0.001 | 58 0.0 (48.0–67.0) | 81.0 (70.0–100.0) | 0.003 | 56.0 (49.0–67.0) | 70 (60–76) | <0.001 |
| Scr on ICU admission (μmol/L) | 56.8 (48.6–68.8) | 83.3 (67.3–123.8) | <0.001 | 55.6 (47.9–67.7) | 100.2 (70.7–121.9) | 0.002 | 61.0 (48.9–71.5) | 82.6 (61.8–124.5) | <0.001 |
| Scr at the time of AKI diagnosis (μmol/L) | 56.8 (48.6–68.8) | 127.5 (111.3–161.2) | <0.001 | 55.6 (47.9–67.7) | 104.9 (101.1–161.2) | <0.001 | 61.0 (48.9–71.5) | 127.7 (112.2–161.1) | <0.001 |
| Peak Scr (μmol/L) | 67.6 (57.6–77.1) | 183.6 (126.1–279.5) | <0.001 | 67.6 (55.8–77.4) | 139.4 (104.6–181.7) | <0.001 | 66.8 (60.4–77.9) | 212.5 (129.6–306.7) | <0.001 |
| Oliguria | – | 17 (35.4) | <0.001 | – | – | – | – | 17 (41.5) | <0.001 |
| AKI Stage | <0.001 | <0.001 | <0.001 | ||||||
| 1 | – | 17 (35.4) | – | – | 6 (85.7) | – | – | 11 (26.8) | – |
| 2 | – | 9 (18.8) | – | – | 1 (14.3) | – | – | 8 (19.5) | – |
| 3 | – | 22 (45.8) | – | – | 0 | – | – | 22 (53.7) | – |
Note: Values are n (%), mean ± SD or median (interquartile range).
Abbreviations: AKI, acute kidney injury; MAP, mean arterial pressure, 1 mmHg=0.133 kPa; ICU, intensive care unit; Scr, serum creatinine; BUN, blood urea nitrogen.
On ICU admission, 43.0% (46/107) of patients had proteinuria, and 43.9% (47/107) had hematuria. The peak stages of 48 AKI cases were stage 1 in 35.4% (17/48), stage 2 in 18.8% (9/48), and stage 3 in 45.8% (22/48) (Table 2). During follow-up, a total of 51 patients (47.7%) died, including 40 patients with AKI and 11 without AKI, and 56 (52.3%) were discharged.
Table 2.
Complications, Treatments and Outcomes of Patients Between AKI and Non-AKI with Different Severities of Coronavirus Disease 2019
| Characteristics | All Patients (n=107) | P-value | Severe Patients | P-value | Critical Patients | P-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-AKI (59, 55.1) | AKI (48, 44.9) | Non-AKI (30, 81.1) | AKI (7, 18.9) | Non-AKI (29, 55.1) | AKI (41, 44.9) | ||||
| Complications | |||||||||
| Acute respiratory distress syndrome | 19 (32.2) | 29 (60.4) | 0.004 | 3 (10.0) | 1 (14.3) | 0.750 | 16 (55.2) | 28 (68.3) | 0.263 |
| Hypoproteinemia | 22 (37.3) | 19 (39.6) | 0.808 | 8 (26.7) | 3 (42.9) | 0.410 | 14 (48.3) | 16 (39.0) | 0.441 |
| Septic shock | 9 (15.3) | 29 (60.4) | <0.001 | 0 | 0 | – | 9 (31.0) | 29 (70.7) | 0.001 |
| Disseminated intravascular coagulation | 3 (5.1) | 6 (12.5) | 0.169 | 0 | 0 | – | 3 (10.3) | 6 (14.6) | 0.594 |
| Treatment | |||||||||
| Antibiotic therapy | 55 (93.2) | 46 (95.8) | 0.554 | 26 (86.7) | 5 (71.4) | 0.353 | 29 (100.0) | 41 (100.0) | – |
| Glucocorticoids | 51 (86.4) | 40 (83.3) | 0.654 | 25 (83.3) | 3 (42.9) | 0.035 | 26 (89.7) | 37 (90.2) | 0.936 |
| Intravenous immunoglobulin therapy | 32 (54.2) | 29 (60.4) | 0.521 | 16 (53.3) | 2 (28.6) | 0.231 | 16 (55.2) | 27 (65.9) | 0.366 |
| Need for vasopressors | 9 (15.3) | 29 (60.4) | <0.001 | 1 (3.3) | 1 (14.3) | 0.305 | 8 (27.6) | 28 (68.3) | 0.001 |
| Oxygen therapy | 57 (96.6) | 42 (87.5) | 0.072 | 29 (96.7) | 6 (85.7) | 0.305 | 28 (96.6) | 36 (87.8) | 0.174 |
| Non-invasive Mechanical ventilation | 26 (44.1) | 41 (85.4) | <0.001 | 3 (10.0) | 2 (28.6) | 0.232 | 23 (79.3) | 39 (95.1) | 0.040 |
| Invasive Mechanical ventilation | 16 (27.1) | 39 (81.3) | <0.001 | 0 | 1 (14.3) | 0.063 | 16 (55.2) | 38 (92.7) | <0.001 |
| Continuous renal replacement therapy | 3 (5.1) | 17 (35.4) | <0.001 | 0 | 1 (14.3) | 0.063 | 3 (10.3) | 16 (39.0) | 0.008 |
| Extracorporeal membrane oxygenation | 1 (1.7) | 3 (6.3) | 0.212 | 0 | 0 | – | 1 (3.4) | 3 (7.3) | 0.479 |
| Time from symptom onset to hospital admission (days) | 16 (12–26) | 14 (8–20) | 0.010 | 18 (13–26) | 9 (7–22) | 0.010 | 16 (12–35) | 14 (8–20) | 0.055 |
| Time from symptom onset to ICU admission (days) | 21 (14–36) | 19 (14–28) | 0.219 | 21 (14–38) | 17 (9–32) | 0.219 | 21 (14–41) | 19 (14–26) | 0.269 |
| Length of hospital stay (days) | 26 (16–40) | 14 (9–29) | 0.001 | 30 (18–44) | 12 (10–22) | 0.001 | 25 (14–38) | 14 (7–30) | 0.064 |
| Length of ICU stay (days) | 9 (5–15) | 7 (3–13) | 0.442 | 7 (4–9) | 4 (3–10) | 0.442 | 13 (7–18) | 9 (4–23) | 0.201 |
| In-hospital mortality | 11 (18.6) | 40 (83.3) | <0.001 | 0 | 2 (28.6) | 0.007 | 11 (37.9) | 38 (92.7) | <0.001 |
Note: Values are n (%) or median (interquartile range).
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit.
Comparison of AKI Incidence According to COVID-19 Disease Classification
For severe patients, AKI occurred in 7 (18.9%) patients: the proportions with stages 1, 2, and 3 AKI were 85.7%, 14.3%, and 0%, respectively. For critical patients, AKI occurred in 41 (44.9%) patients, and the respective proportions were 26.8%, 19.5%, and 53.7%.
Among the 107 patients, patients with AKI were older; had a higher body mass index (BMI); had a higher prevalence of cardiovascular disease, cerebrovascular disease and CKD; and were accompanied by higher rates of ARDS and septic shock than patients without AKI. Patients with AKI were more frequently treated with noninvasive mechanical ventilation, mechanical ventilation, vasopressors, and CRRT and suffered from proteinuria, hematuria and oliguria. They also had significantly higher blood urea nitrogen (BUN) and uric acid levels as well as higher creatine kinase, lactate dehydrogenase, creatine kinase isoenzyme, cystatin C, D-dimer, C-reactive protein, procalcitonin, and lactate levels at the time of admission than patients without AKI. Low CO2, platelets, and oxygenation index were more common in patients with AKI. Indeed, baseline Scr, Scr on ICU admission, Scr on AKI diagnosis, and peak Scr levels during the hospital stay differed significantly between the 2 groups. Patients with AKI presented higher in-hospital mortality rates (83.3% vs 18.6%, P<0.001) and more frequently exhibited stage 3 AKI.
Furthermore, among the 37 patients in the severe group, patients who developed AKI were older and were more likely to have cerebrovascular disease and CKD than patients without AKI. Patients with AKI were less likely to need glucocorticoids. They also had significantly higher uric acid levels and higher cystatin C levels at the time of admission than patients without AKI. Low leukocytes and platelets were more common in patients with AKI. Indeed, baseline Scr, Scr on ICU admission, Scr on AKI diagnosis, and peak Scr levels during the hospital stay differed significantly between the 2 groups. Patients with AKI presented higher in-hospital mortality rates (28.6% vs 0%, P=0.007) and more frequently exhibited stage 1 AKI.
Indeed, among the 70 patients in the critical group, patients with AKI more frequently were treated with noninvasive MV, invasive MV, vasopressors, and CRRT and suffered from septic shock and proteinuria. They also had significantly higher BUN and uric acid levels as well as higher lactate dehydrogenase, cystatin C, D-dimer, and procalcitonin levels at the time of admission than patients without AKI. Low CO2 and low platelets were more common in patients with AKI. Indeed, baseline Scr, Scr on ICU admission, Scr on AKI diagnosis, and peak Scr levels during the hospital stay differed significantly between the 2 groups. Patients with AKI presented higher in-hospital mortality rates (92.7% vs 37.9%, P<0.001) and more frequently exhibited stage 3 AKI.
Association of AKI and In-Hospital Mortality in COVID-19 Patients
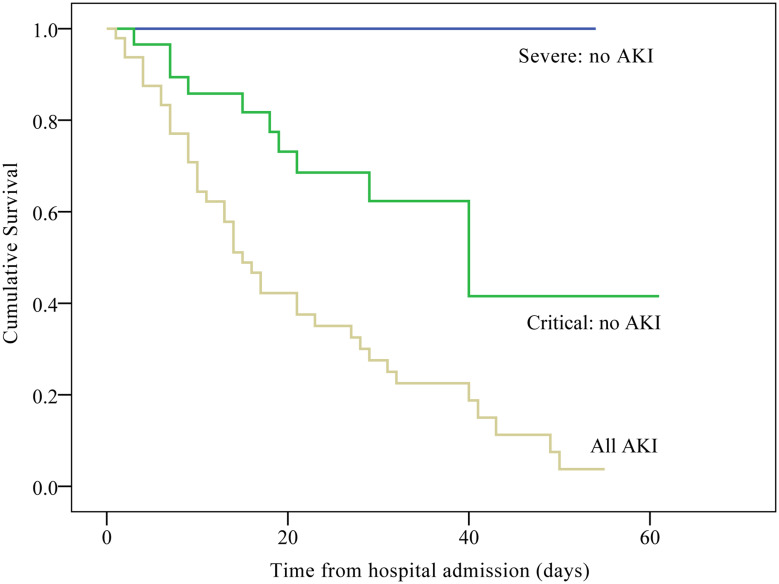
Kaplan–Meier curves showed that survival was better in the non-AKI severe group than in the non-AKI critical group (Log rank all P < 0.001; Figure 2); the hospital survival rate decreased with disease progression. The survival of AKI patients was worse than that of patients with severe and critical disease who did not develop AKI (Log rank all P < 0.001; Figure 2). After adjusting for BMI, diabetes mellitus, muscle ache, disease classification, albumin, aspartate aminotransferase, BUN, creatine kinase, lactate dehydrogenase, creatine kinase isoenzyme, CO2, D-dimer, leukocytes, platelets, C-reactive protein, lactate, PH, PaCO2, PaO2, baseline Scr, Scr on ICU, peak Scr, ARDS, disseminated intravascular coagulation, oxygen therapy, need for vasopressors, and noninvasive MV, the multivariable adjusted logistic proportional hazard regression model showed a significantly higher risk of in-hospital death in COVID-19 patients with AKI than in those without AKI (OR = 33.74; 95% CI = 3.34–341.29; P = 0.003). The other independent risk factors for in-hospital mortality included septic shock (OR = 15.58; 95% CI = 2.08–116.78; P = 0.008), invasive MV (OR = 18.44; 95% CI = 2.35–144.69; P = 0.006), and oxygenation index (OR = 0.99; 95% CI = 0.98–1.000; P = 0.014) (Table 3).
Figure 2.
Kaplan–Meier survival curves for patients with severe to critical COVID-19 with and without AKI during the time from hospital admission (Log rank test P < 0.001).
Table 3.
Univariable and Multivariable Logistic Regression Analyses for In-Hospital Mortality with Coronavirus Disease 2019
| Charcteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| AKI | 21.81 (8.00–59.46) | <0.001 | 33.74 (3.34–341.29) | 0.003 |
| Invasive mechanical ventilation | 112.00 (27.35–458.68) | <0.001 | 18.44 (2.35–144.69) | 0.006 |
| Septic shock | 64.80 (13.97–300.61) | <0.001 | 15.58 (2.08–116.78) | 0.008 |
| Oxygenation index | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.98–1.00) | 0.014 |
| Body mass index | 1.36 (1.11–1.68) | 0.004 | ||
| Diabetes mellitus | 2.92 (1.08–7.89) | 0.035 | ||
| Muscle ache | 3.03 (1.32–6.96) | 0.009 | ||
| Critical vs severe | 40.83 (8.99–185.56) | <0.001 | ||
| Albumin | 0.85 (0.76–0.94) | 0.002 | ||
| Aspartate aminotransferase | 1.02 (1.00–1.04) | 0.012 | ||
| BUN | 1.22 (1.08–1.37) | 0.001 | ||
| Creatine kinase | 1.00 (1.00–1.01) | 0.035 | ||
| Lactate dehydrogenase | 1.01 (1.00–1.01) | <0.001 | ||
| Creatine kinase isoenzyme | 1.18 (1.09–1.28) | <0.001 | ||
| CO2 (mmol/L) | 0.89 (0.81–0.97) | 0.011 | ||
| D-dimer (mg/L) | 1.11 (1.02–1.21) | 0.013 | ||
| Leucocytes | 1.08 (1.01–1.15) | 0.015 | ||
| Platelets | 0.99 (0.99–1.00) | 0.003 | ||
| C-reactive protein | 1.01 (1.01–1.02) | <0.001 | ||
| Lactate | 2.47 (1.58–3.87) | <0.001 | ||
| PH | 0.00 (0.00–0.17) | 0.007 | ||
| PaCO2 | 1.05 (1.02–1.09) | 0.006 | ||
| PaO2 | 0.98 (0.97–0.99) | 0.003 | ||
| Baseline Scr | 1.04 (1.01–1.07) | 0.013 | ||
| Scr on ICU | 1.02 (1.00–1.03) | 0.018 | ||
| Peak Scr | 1.02 (1.01–1.03) | <0.001 | ||
| Acute respiratory distress syndrome | 7.24 (3.07–17.05) | <0.001 | ||
| Disseminated intravascular coagulation | 10.23 (1.23–84.98) | 0.031 | ||
| Oxygen therapy | 0.11 (0.01–0.96) | 0.046 | ||
| Need for vasopressors | 38.64 (10.48–142.49) | <0.001 | ||
| Non-invasive mechanical ventilation | 51.72 (11.30–236.74) | <0.001 | ||
Abbreviations: AKI, acute kidney injury; BUN, blood urea nitrogen; ICU, intensive care unit; Scr, serum creatinine; OR, odds ratio; CI, confidence interval.
Independent Predictors of AKI
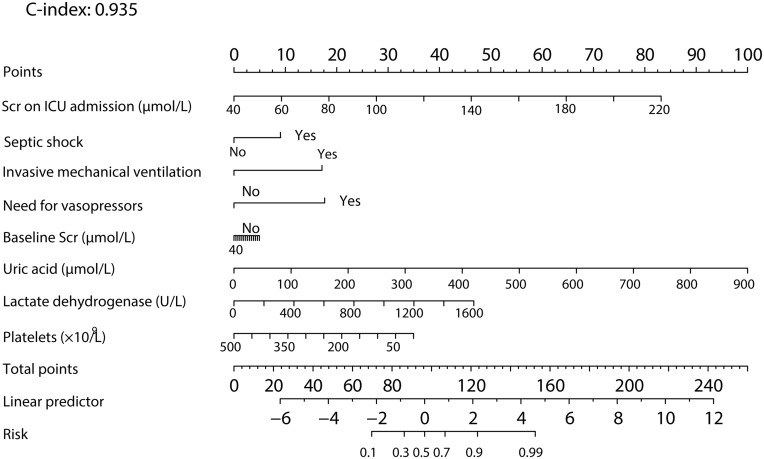
A prediction model for the occurrence of AKI was constructed based on the multivariate model. Independent predictors of incident AKI in patients who developed AKI while in the ICU were baseline Scr, septic shock, invasive MV, need for vasopressors, and Scr on ICU admission, as well as uric acid, lactate dehydrogenase and platelets, based on the results of lasso logistic regression. We included these factors in multivariate logistic regression to develop a prediction model for AKI. That is, logit (P)= −19.51–0.66×baseline Scr+0.57×Scr on ICU admission +0.04×uric acid+0.0044×lactate dehydrogenase-0.0083×platelets −0.95×septic shock+9.39×invasive MV+1.38×need for vasopressors. P indicates the possibility of AKI. Baseline Scr, Scr at admission and uric acid are the values of laboratory tests, and the unit is μmol/L; the unit of platelets is ×109/L. The unit of lactate dehydrogenase is U/L. For septic shock, a value of 1 means the patients experienced septic shock, whereas 0 means there was no occurrence of septic shock. For invasive MV and the need for vasopressors, a value of 1 means the treatments were used, whereas 0 means the treatment was not used. A nomogram was presented based on the prediction model (Figure 3); based on the nomogram, we first scored each factor based on the points scale at the top of the nomogram and then summed the points of each factor. Finally, the AKI onset probability was obtained based on the bottom point scale of the nomogram.
Figure 3.
Prediction nomogram for predicting the AKI onset probability of patients with coronavirus disease 2019. Prediction of the patient’s value is located on each variable axis, and a line is drawn upward to determine the number of nomogram points for predicting the AKI probability of patients with COVID-19. The sum of these numbers is located on the total points axis, and a line is drawn downward to the predicted axes to determine the likelihood of AKI (C-index: 0.935; 95% CI, 0.892–0.978).
The C-index for the development of the AKI model was 0.935 (95% CI, 0.892–0.978). We further used 10-fold cross validation to validate the model, showing that the corrected C-index was 0.825 and indicating that the model has good generalizability for patients with COVID-19 in the ICU.
Discussion
The present retrospective study demonstrates the statistically significant association between AKI and in-hospital mortality in ICU patients with COVID-19. Although COVID-19 patients have a high risk of developing AKI, there is no information regarding the incidence and clinical significance of AKI in severely versus critically ill patients. The present study adds several novel findings by exploring the relationship between AKI and mortality. First, AKI is a common complication in COVID-19 ICU patients, and more than 44% of inpatients develop AKI during their hospital stay. Second, as COVID-19 pneumonia progresses, the incidence of AKI increases significantly; this proportion is 19% in severe patients and increases to 45% in critical patients. Third, compared with that of severely or critically ill patients without AKI, the in-hospital mortality was higher in the AKI patients. This phenomenon suggests that AKI can be viewed as a marker of disease severity and a negative prognostic factor for survival.
The etiology of AKI in patients with COVID-19 is likely to be multifactorial. First, the novel coronavirus may exert direct cytopathic effects on kidney tissue. Recent human tissue RNA-sequencing data have demonstrated that angiotensin-converting enzyme 2 (ACE2) expression in the kidney is much higher than that in the lungs. Therefore, AKI may be caused by coronavirus entering kidney cells through an ACE2-dependent pathway. Second, deposition of immune complexes of viral antigen or virus-induced specific immunological effector mechanisms may damage the kidney. Third, virus-induced cytokines or mediators might exert indirect effects on renal tissue, such as hypoxia, shock, and rhabdomyolysis. However, other risk factors may also contribute to AKI onset, particularly in critically ill patients. First, most COVID-19 patients with AKI are older and have frequent comorbidities, such as hypertension; these factors are well-known factors of kidney injury. Second, the fundamental pathophysiology of pneumonia in critically ill patients is severe ARDS, and impairment of gas exchange and severe hypoxemia (with a median oxygenation index at 130 [77–255] mmHg at ICU admission in our cohort) have been recognized as factors associated with AKI. Third, the pathophysiology of AKI in the ICU currently relies on unspecific mechanisms, such as septic shock, hypovolemia and subsequent prerenal AKI, nephrotoxic drugs, injurious MV strategies such as permissive hypercapnia or permissive hypoxemia and positive pressure ventilation and a high level of positive end-expiratory pressure.
Multiple previous observational studies have been published, and the incidence of AKI is scant, varying from 8.3% to 68% in ICU patients13,22–25 and 0% to 46% in non-ICU patients.6,9,10,22,25–28 However, we cannot completely explain this difference. One possible explanation is the design of the study or the AKI definitions. For example, one multicenter 1-day cross-sectional prospective study by Yu et al, which assessed 19 ICUs with a median age of 64 years in Wuhan, found that AKI occurred in 25.2% (57/226) of patients.23 Interestingly, another prospective observational cohort of 257 COVID-19 critically ill patients in New York City lacked data on AKI.29 Importantly, the present method of detecting AKI is mainly based on acute changes in Scr, and the frequency of Scr tests has a substantial impact on the detection rate. Therefore, the familiarity and awareness of the definition of AKI by clinicians also determine the incidence or recognition rate of AKI. Thus, to improve the early detection of AKI, more frequent Scr measurements should be performed in patients with COVID-19.
MV is the main supportive treatment for severe patients. We found that patients with AKI were more likely to undergo MV and that the oxygenation index was low in such patients, suggesting that AKI during the hospital stay represented an increased risk of deterioration. Endotracheal intubation and invasive MV were needed in 51.4% of the patients, whereas the proportion that needed invasive MV was as high as 81.3% in AKI patients. The need for invasive MV in our cohort was higher than that recently reported for other ICU patients: 47.2% (Wuhan, China),22 42% (Wuhan, China),24 37.6% (Wuhan, China),23 and 15% (Wuhan, China),25 and higher than 2 Chinese studies of COVID-19 severe or critical patients (with rates of 31.5% at three hospitals in Wuhan, Shanghai, and Anhui, China,30 and 21.2% in Wuhan, China,31) but lower than the rate of 88% reported in the Lombardy region, Italy,3 71~75% in Washington and 79% of New York, US,4,5,29 and 62.5% in a nationwide analysis in China.32 The higher proportion of patients requiring invasive MV in our cohort was due to acute hypoxemic respiratory failure that required respiratory support. The oxygenation index was lower in our patients than in patients admitted in Italy, where the median level of oxygenation index at ICU admission was 160 (114–220),3 but similar to that of patients admitted in the USA, with an oxygenation index of 129 (80–203),29 as the median oxygenation index in our population was 130 (77–255), indicating that this level is associated with the severity of illness and thus prognosis.
It has been reported previously that AKI was associated with an increased risk of death in patients with SARS and MERS. In-hospital mortality in patients with COVID-19 was 47.7% overall, including 83.3% of patients who experienced AKI and 18.6% of patients without AKI. Indeed, in-hospital mortality in patients with AKI was 28.6% in severe patients and 92.7% in critical patients. A previous study reported different mortality rates in severely and critically ill patients, from 16.7%,22 to 26.0%,3,30 35.0%,12 39.0%,29 43.6%,33 50.0%,4 61.5%,24 and 67.0%.3 In a recent observational, retrospective study of 1406 AKI patients with COVID-19 in New York City, in-hospital mortality was 41% overall and 52% in the ICU.13 The mortality rates increased if the follow-up time was prolonged. However, the criteria for ICU admission were different among the studies, which was another reason for the different mortality rates.
Severely or critically ill patients have a high risk of developing AKI. When AKI develops, AKI is usually an indicator of more severe disease and multiorgan dysfunction, so kidney function protection should be an important part of clinical treatment. The study first developed a nomogram model to accurately predict AKI onset in ICU patients with COVID-19 based on individual characteristic risk factors. The predictive nomogram performed well in predicting AKI, supported by the C-index (0.935; 95% CI, 0.892–0.978). In our study, CRRT was used in 14.3% of severe AKI patients, compared with 39.0% of critically ill patients during the study. One unexpected finding was that 17 (35.4%) AKI patients and 3 (5.1%) non-AKI patients who underwent CRRT died. Future studies should investigate the early initiation of RRT and sequential extracorporeal therapies as a means to provide adequate organ support and to prevent the progression of COVID-19 severity. Classical assessment of AKI is still based on Scr and urine output, but they represent indicators of only established kidney damage.
As AKI was an independent risk factor for in-hospital mortality, we developed a prediction model for the occurrence of AKI. Based on baseline Scr, septic shock, invasive MV, need for vasopressors, and Scr on ICU admission, we can predict AKI with a C-index of 0.935. When validating the model using 10-fold cross validation, the C-index still reached 0.825, which means good generalizability and performance of the model we developed. Awareness of these characteristics may be a useful tool to help physicians predict AKI during hospital stay.
The study had the following limitations. (1) First, this is a single-center retrospective study, and a total of 107 severely and critically ill patients admitted to the ICU were included. However, the population from which they were sampled was much larger than that of the three studies previously published.22,24,25 We included all severe patients who were cared for in the ICU of Huo Shen Shan Hospital and met the inclusion criteria. Due to the exploratory nature of the study, which was not driven by formal hypotheses, sample size calculation was waived. Instead, we hope that the findings presented here will encourage a larger cohort study or potentially some randomly controlled trials. (2) Second, some specific information from the ICU was incomplete, such as urine output, which is one component of the KDIGO definition; therefore, the incidence of AKI might have been underestimated. Additionally, in some cases, there was incomplete documentation of the history or laboratory findings in the electronic database even after making efforts regarding feedback and recollection. Some diagnoses of coexisting illness were from patients’ self-reports at admission, which might lead to recall bias. Although the cohort had broad coverage of all patients and regions, nonresponse bias cannot be fully excluded. (3) Third, we lacked data from the time after discharge. Therefore, we could not assess the effects of COVID-19 on long-term survival and kidney function.
Conclusion
The prevalence of AKI among COVID-19 patients admitted to the ICU was high. AKI is considered a marker of disease severity and a negative prognostic factor for in-hospital survival. The nomogram proposed in this study objectively predicted AKI development in COVID-19 patients in the ICU. Therefore, the strategy for kidney protection against severe or critical pneumonia is appropriate.
Acknowledgments
This manuscript was edited for English language by American Journal Experts (AJE).
Funding Statement
This study was funded by grants from the National Natural Science Foundation of China (grant 81871587 to Dr FHZ; grant 81970383 to Dr YC), China National Defense Technology Innovation Project (grant 20-163-12-ZD-027-003-06 to Dr FHZ), the Military Medical Innovation Project (grant 18CXZ026 to Dr FHZ), and the Special Scientific Research Project of Military Health Care (grant 20BJZ27 to Dr FHZ).
Abbreviations
COVID-19, coronavirus disease 2019; SARS, severe acute respiratory syndrome; MERS, Middle East respiratory syndrome; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; AKI, acute kidney injury; KDIGO, Kidney Disease, Improving Global Outcomes; CRRT, continuous renal replacement therapy.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethical Approval
This study was approved by the National Health Commission of China and the Institutional Review Board at Huo Shen Shan Hospital (HSSLL028, Wuhan, China).
Informed Consent
The requirement for written informed consent was waived by the ethics committee of the designated hospital for patients with emerging infectious diseases.
Disclosure
The authors declare that they have no competing interests.
Qinglin Li, Tianyi Zhang and Fei Li contributed equally to this work. Ling Tao, Feihu Zhou and Yue Cai contributed equally to this work.
References
- 1.Gabarre P, Dumas G, Dupont T, et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348. doi: 10.1007/s00134-020-06153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically Ill patients in the Seattle Region - case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8(7):738–742. doi: 10.1016/S2213-2600(20)30229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348. doi: 10.1159/000507471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan L, Chaudhary K, Saha A, et al. Acute kidney injury in hospitalized patients with COVID-19. medRxiv. 2020. [Google Scholar]
- 14.Chu KH, Tsang WK, Tang CS, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkindi F, Boobes Y, Chandrasekhar Nair S, et al. SAT-028 acute kidney injury associated with middle east respiratory syndrome coronavirus (MERS-CoV) infection. Kidney Int Rep. 2020;5(3):S13. doi: 10.1016/j.ekir.2020.02.033 [DOI] [Google Scholar]
- 16.Chao CT, Tsai HB, Wu CY, et al. The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep. 2015;5(1):13925. doi: 10.1038/srep13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Wang J, Su T, et al. Community-acquired acute kidney injury: a nationwide survey in China. Am J Kidney Dis. 2017;69(5):647–657. doi: 10.1053/j.ajkd.2016.10.034 [DOI] [PubMed] [Google Scholar]
- 18.Cheng X, Wu B, Liu Y, et al. Incidence and diagnosis of acute kidney injury in hospitalized adult patients: a retrospective observational study in a tertiary teaching Hospital in Southeast China. BMC Nephrol. 2017;18(1):203. doi: 10.1186/s12882-017-0622-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei JF, Huang FY, Xiong TY, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106(15):1154–1159. doi: 10.1136/heartjnl-2020-317007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Xu D, Fu S, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):219. doi: 10.1186/s13054-020-02939-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]