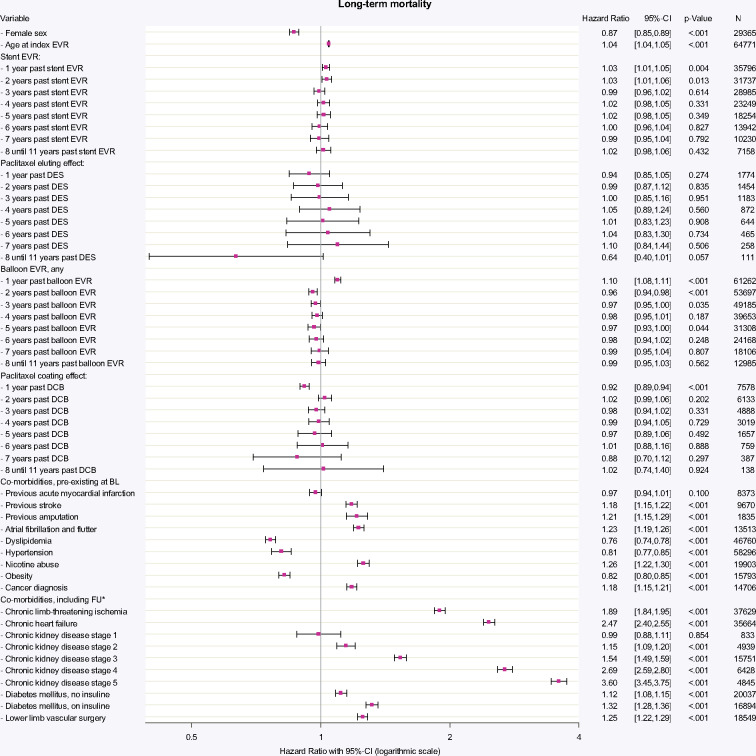
Figure 2.
Time-dependent Cox-regression allowing for non-linear and time-dependent hazard ratio of the respective devices. All devices applied between 1 January 2007 and 31 December 2017 were included in the model. HRs for each type of device are given per application of one distinct device over annual time intervals. HR for stent EVR (any device) was increased for the first 2 years (both HR 1.03; P = 0.004 and P = 0.013), reflecting the procedural risk and general hazard being involved with the need for stent implantation. The additional effect by use of paclitaxel-based DES was not associated with increased mortality in up to 11 years past application. Likewise, balloon EVR (any device) was associated with evident increased mortality in the first year of application (HR 1.10; P < 0.001). Paclitaxel coating effect in DCB was associated with a protective effect in the first year of DCB application (HR 0.92; P < 0.001), which became irrelevant in the years thereafter. The HR of any combination of applied devices including use of multiple devices in different years and concomitant risk factors can be calculated by multiplying elementary HRs: e.g. a patient with previous stroke, diabetes without insulin and one DES 1 year ago plus two POBA 3 years ago compared to a patient without stroke, without diabetes, only one POBA 3 years ago and identical baseline characteristics (e.g. age and gender) has a HR = [1.18 (previous stroke) * 1.12 (diabetes w/o insulin) * 1.03 (1 stent one year ago) * 0.94 (DES) * 0.972 (two POBAs 3 years ago)]/[0.97 (1 POBA three years ago)] = 1.20/0.97 = 1.24. BL, baseline; CI, confidence interval; DCB, drug-coated balloon; DES, drug-eluting stent; EVR, endovascular revascularization; FU, follow-up period; N, number of patients at end of FU. aTime-dependent adjustment of covariates.