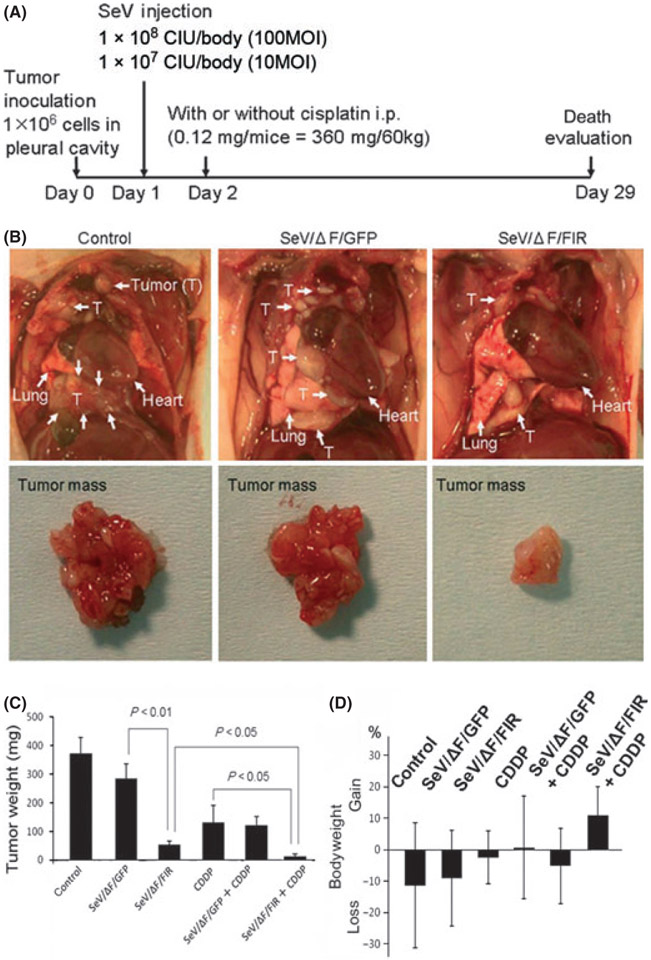
Fig. 3.
(A) Design of the animal experiment used to examine the effect of fusion gene deleted non-transmissible Sendai virus vector encoding FIR (SeV/ΔF/FIR) in an orthotopic xenografted mouse model. 211H cells were injected into the pleural cavity with or without cisplatin. Sendai virus (SeV) was injected into the intrathoracic cavity on day 1 after tumor cell inoculation. Intraperitoneal administration (i.p.) of cisplatin (0.12 mg/body) was performed on day 2. (B) Representative photographs of tumors from control (PBS) and SeV (1 × 108 cell-infectious units [CIU]/body) treatment groups on day 29. (C) Weights of tumors treated with SeV (1 × 108 CIU/body) with or without cisplatin (CDDP) in vivo (n = 10). Significant reductions in tumor weight were observed on day 29 (error bars: SD). (D) Bodyweight changes among mice during SeV treatment (1 × 108 CIU/body) on day 29. Control (PBS), SeV/ΔF/FIR and fusion gene deleted non-transmissible Sendai virus vector encoding green fluorescent protein (SeV/ΔF/GFP) groups (n = 7 per group). Cisplatin (CDDP), SeV/ΔF/FIR plus CDDP, and SeV/ΔF/GFP plus CDDP groups (n = 9 per group) (error bars, SD).