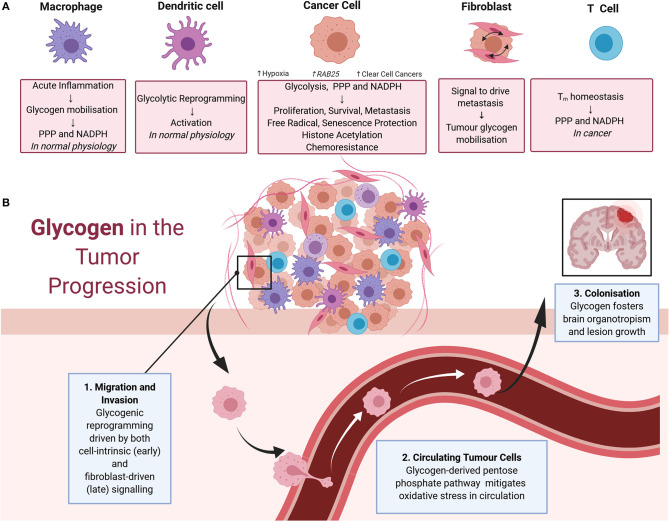
Figure 2.
(A) Impacts of glycogen on cells of the tumor microenvironment. In macrophages, physiologically acute inflammation causes glycogen mobilization as a feedstock for the pentose phosphate pathway and NADPH production. Glycogen also drives the early stages of dendritic cell activation via glycolytic reprogramming. These physiological processes are also thought to contribute to cancer progression. In cancer cells, glycogen fuels glycolysis, the pentose phosphate pathway, and NADPH production to support cell proliferation, survival, metastasis, free radical protection, cell senescence protection, histone acetylation, and chemoresistance. Tumor glycogen metabolism increases in a variety of scenarios, including in hypoxic conditions and via the induction of the RAB25 oncogene. Tumor glycogen metabolism is also ubiquitously high in clear cell cancers. In cancer associated fibroblasts, bi-directional signaling between fibroblasts, and neighboring cancer cells drives tumor glycogen mobilization to fuel metastasis. For T cells, glycogen stores consumed via the pentose phosphate pathway for NADPH production are essential for CD8+ memory T cell survival. (B) Glycogen in tumor progression. Glycogen mobilization is implicated in metastasis including as fuel for: (1) migration and invasion of cancer cells in the early stages of metastasis with crosstalk between fibroblasts and malignant cells also eliciting glycogenolysis to fuel migration and invasion; (2) the pentose phosphate pathway in circulating malignant cells facilitating detoxification of reactive oxygen species and promotion of survival; and (3) colonization of distant sites including in the survival of brain metastases.