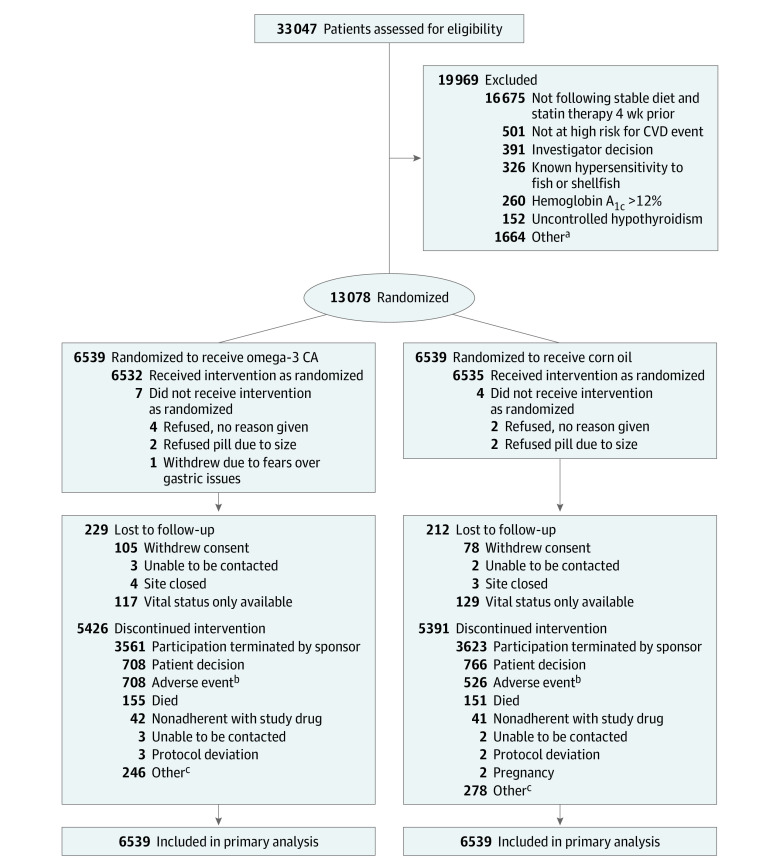
Figure 1. Recruitment, Randomization, and Patient Flow in the STRENGTH Clinical Trial .
CA indicates carboxylic acid formulation; CVD, cardiovascular disease.
aOther reasons for not meeting inclusion/exclusion criteria include not meeting age requirement; elevated liver enzymes; use of fibrates, bile acid sequestrants, or niacin within 4 weeks of randomization; not following a stable diet; poorly controlled hypertension; and occurrence of myocardial infarction or coronary bypass graft surgery within 30 days of randomization.
bAdverse events leading to study drug discontinuation by system organ class (omega-3 CA/corn oil; multiple events are possible): gastrointestinal (403/202), neoplasms (81/78), cardiac (39/46), nervous system (36/42), infections (32/30), skin (24/20), kidney/urinary (16/25), investigations (21/14), metabolic disorders (18/17), musculoskeletal (14/18), hepatobiliary (13/14), injury (11/13), vascular (13/11), respiratory (13/10), and psychiatric (11/7).
cOther reasons abstracted from free text (omega-3 CA/corn oil): investigator decision (22/22), patient decision (26/33), potential lost to follow-up (113/129), reached end point (18/18), moved (31/36), social reasons (7/13), comorbid condition (11/8), pill burden (5/10), study terminated (9/4), and site closed (4/5).