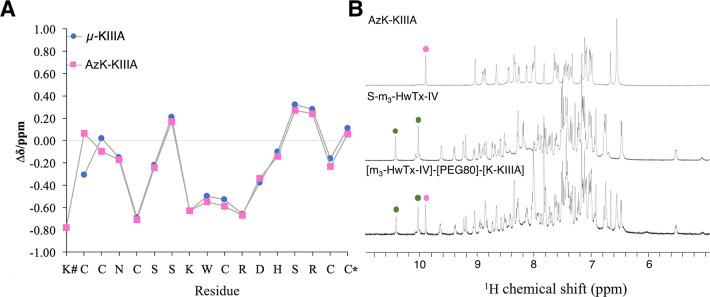
Figure 3.
NMR analysis of AzK-KIIIA, S-m3-HwTx-IV, and [m3-HwTx-IV]-[PEG80]-[K-KIIIA]. (A) Secondary Hα chemical shifts of μ-KIIIA29 and AzK-KIIIA obtained from sequence-specific resonance assignments using two-dimensional TOCSY and NOESY spectra. The secondary Hα shifts aligned throughout Asn3–Cys17. The N-terminal modification with AzK had chemical shift differences of 0.37 ppm for Cys1 Hα and 0.12 ppm for Cys2 Hα. The x-axis shows the sequence of AzK-KIIIA. The hash (#) indicates AzK, and the asterisk (*) indicates C-terminal amidation. (B) 1H NMR spectra [600 MHz, 25 °C, 90/10% (v/v) H2O/D2O] of AzK-KIIIA, S-m3-HwTx-IV, and [m3-HwTx-IV]-[PEG80]-[K-KIIIA]. The spectrum of [m3-HwTx-IV]-[PEG80]-[K-KIIIA] was the sum of the spectra of its individual peptide components as is exemplified for the tryptophan ε-NH region of AzK-KIIIA (magenta dots) and S-m3-HwTx-IV (green dots). Additional signals in the fingerprint region of the conjugate correspond to five secondary amide protons present in the synthetic PEG80 linker, and the C5 proton in the 1,2,3-triazole occurring in the bivalent compound.