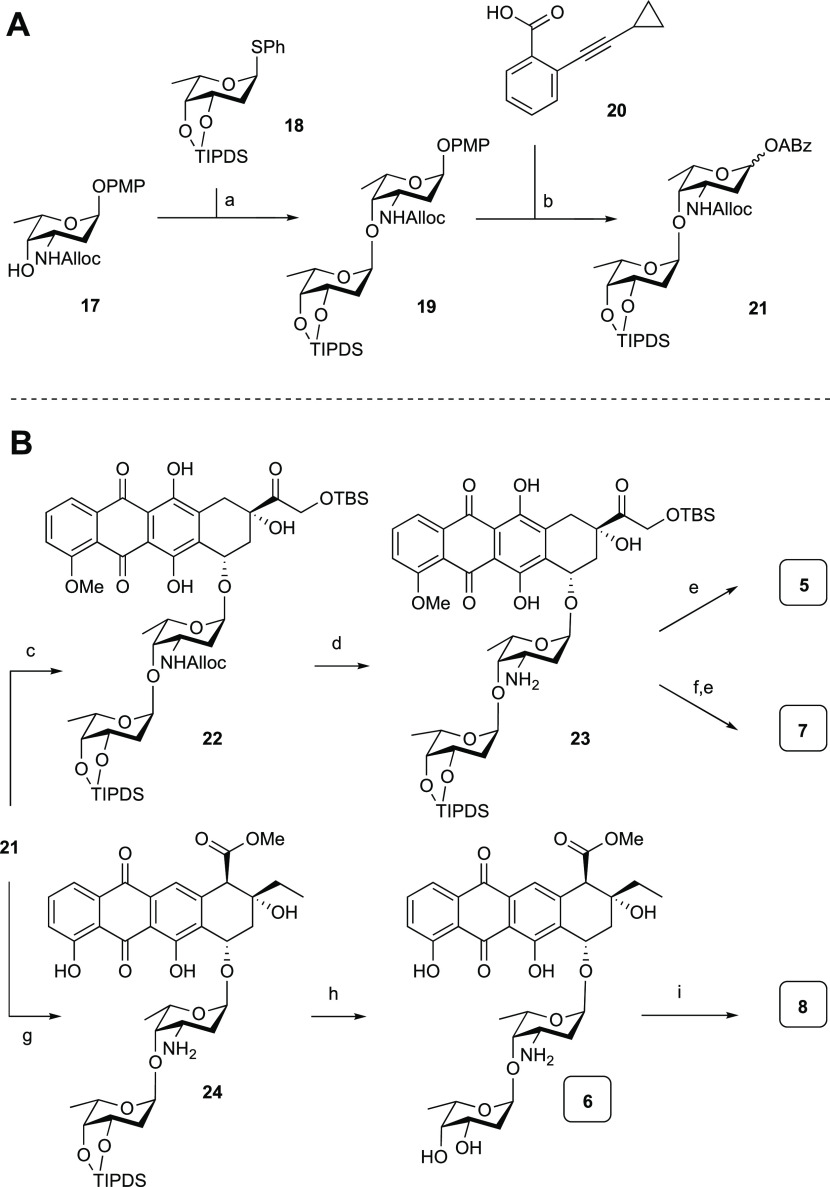
Scheme 2. (A) Synthesis of Disaccharide Alkynylbenzoate Donor 21; (B) Synthesis of Hybrid Disaccharide Anthracyclines 5–8.
Reagents and conditions: (a) IDCP, Et2O, 1,2-dichloroethane (DCE) (4:1 v/v), then PPh3, 89%; (b) (i) Ag(II)(hydrogen dipicolinate)2, NaOAc, MeCN, H2O, 0 °C, (ii) 20, EDCI·HCl, N,N-diisopropylethylamine (DIPEA), 4-dimethylaminopyridine (DMAP), DCM, 84% over two steps (1:8 α/β).
Reagents and conditions: (c) 16, PPh3AuNTf2 (10 mol %), DCM, 64% (>20:1 α/β); (d) Pd(PPh3)4, NDMBA, DCM, quant.; (e) HF·pyridine, pyr., 76% for 5, 81% for 7; (f) aq CH2O, NaBH(OAc)3, EtOH, 71%; (g) (i) 14, PPh3AuNTf2 (10 mol %), −20 °C, DCM, (ii) Pd(PPh3)4, NDMBA, DCM, 87% over two steps (>20:1 α/β); (h) HF·pyridine, pyr., 41%; (i) aq CH2O, NaBH(OAc)3, EtOH, 34%.