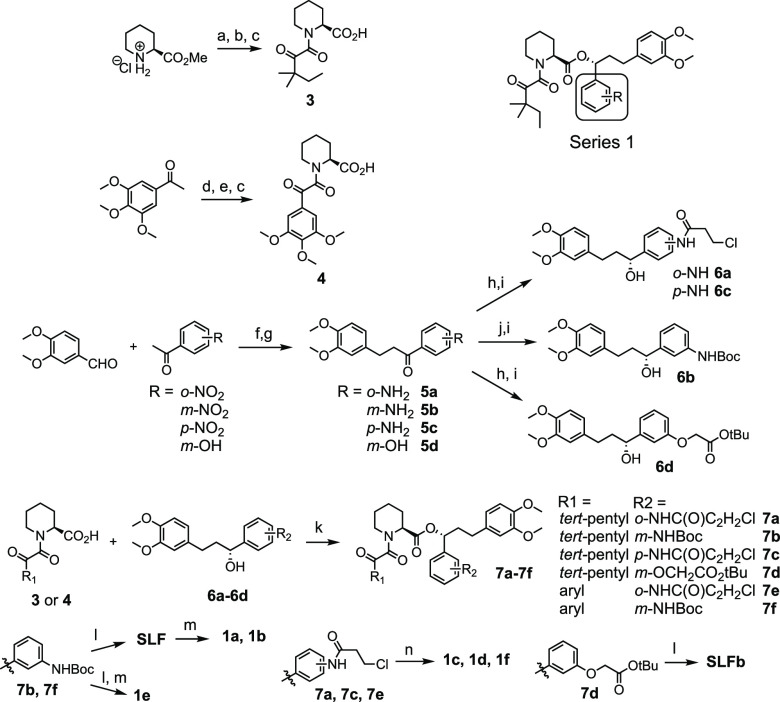
Scheme 1. Synthesis of Series 1 Compounds.
Reagents and conditions: (a) Methyl oxalyl chloride, DIPEA, DCM, 22 °C, 6 h; (b) 1,1-dimethylpropylmagnesium chloride, THF, −78 °C, 2 h; (c) LiOH, MeOH, 0–25 °C, 12 h; (d) SeO2, pyridine, 110 °C, 3 h; (e) oxalyl chloride, DMF, DCM, 0 °C, 30 m then: pipecolic acid methyl ester, NEt3, DCM, 0 °C, 2 h; (f) KOH, EtOH, 0 °C, 12 h; (g) H2, Pd/C, EtOAc, 2–24 h; (h) 3-chloropropionoyl chloride OR tert-butyl bromoacetate, K2CO3, acetone, 22 °C, 16 h; (i) (+)-DIP-chloride, THF, −45 °C, 16 h then: diethanolamine, Et2O, 22 °C, 2 h; (j) Boc2O, 1,4-dioxane, 150 °C, 4 h; (k) DCC, DMAP, DCM, 22 °C, 2 h; (l) TFA, DCM, 22 °C, 2 h; (m) acryloyl chloride OR propionyl chloride, NEt3, DCM, 0 °C, 2 h; (n) NEt3, MeCN, 80 °C, 6–18 h.