Fig. 2 |. Mechanisms of impaired efferocytosis in disease.
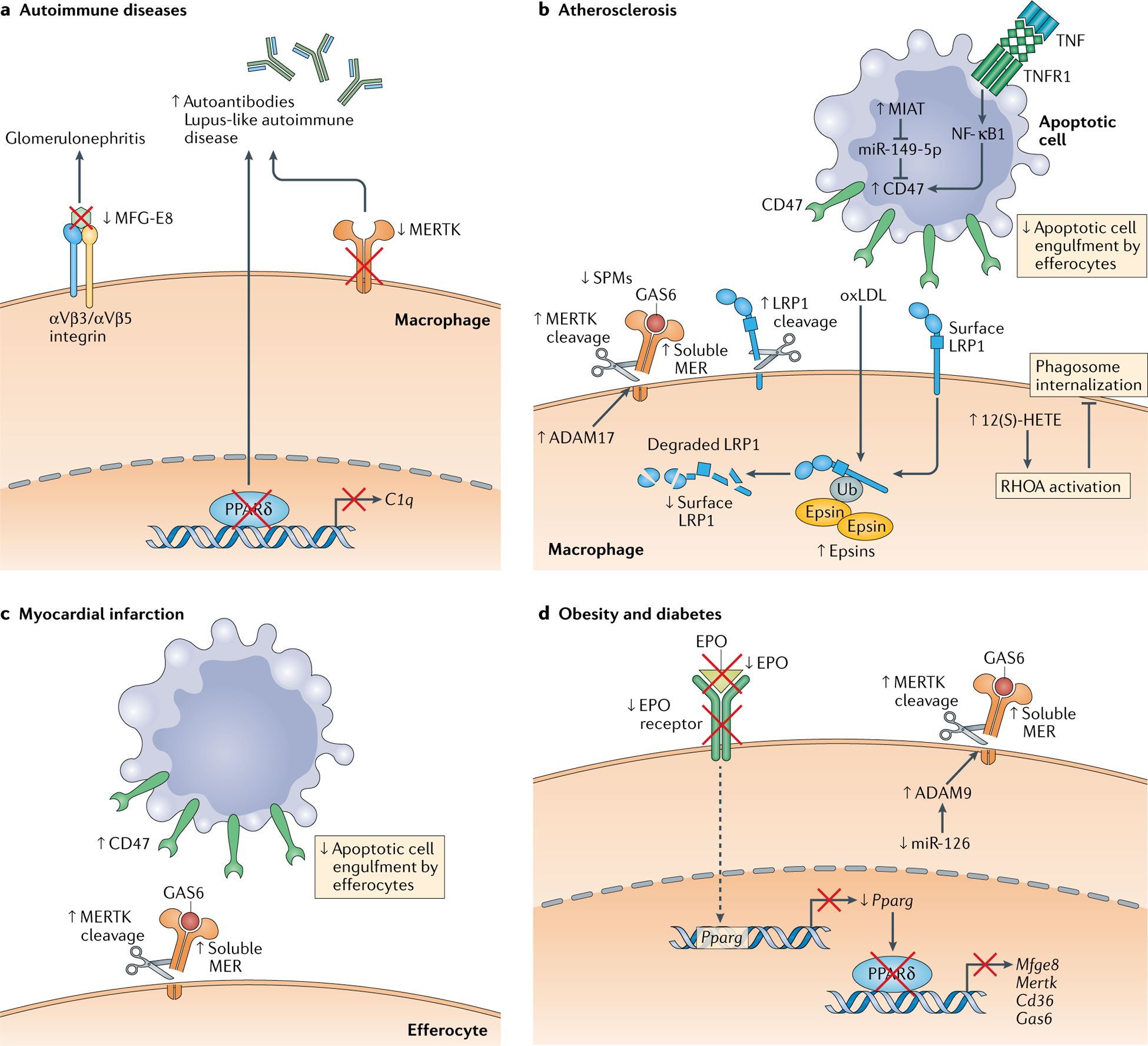
A variety of impairments contribute to defective macrophage efferocytosis and the development and/or progression of disease. a | In the autoimmune disease glomerulonephritis, efferocytosis is impaired due to loss of the efferocytosis bridging molecule milk fat globule-EGF factor 8 (MFG-E8). The development of autoantibodies and lupus-like autoimmune disease in mice is associated with impaired efferocytosis due to reduced levels of the efferocytosis receptor MER proto-oncogene tyrosine kinase (MERTK) or to impaired transcription of the efferocytosis bridging molecule C1q by peroxisome proliferator-activated receptor-δ (PPARδ). b | In atherosclerosis, proteolytic cleavage of the efferocytosis receptors MERTK and low-density lipoprotein receptor-related protein 1 (LRP1) compromises the ability of plaque macrophages to clear dead cells in atherosclerotic lesions, which contributes to plaque necrosis and impaired resolution. The MERTK cleavage product, soluble MER, may be able to compete for the bridging molecule growth arrest-specific protein 6 (GAS6), sequestering it from intact MERTK receptors. Cell-surface LRP1 levels are also decreased by epsin-mediated internalization of the receptor. A particular lipid that accumulates in atherosclerotic lesions called arachidonic acid-derived 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) suppresses apoptotic cell internalization by activating RHOA. Finally, apoptotic cells within atherosclerotic plaques upregulate the ‘don’t-eat-me’ signal CD47, impairing their ability to be recognized by phagocytes. Several mechanisms have been proposed, including induction of CD47 expression by a tumour necrosis factor (TNF)−TNF receptor 1 (TNFR1)−nuclear factor-κB1 (NF-κB1) pathway and loss of a CD47-suppressing microRNA, miR-143–5p. c | Similarly to atherosclerosis, both MERTK cleavage in cardiac macrophages and inappropriately high levels of CD47 on apoptotic cardiomyocytes contribute to an impaired resolution response after experimental myocardial infarction. d | In obesity and diabetes, MERTK cleavage also plays an important role in the impaired ability of diabetic macrophages to clear apoptotic cells, although in contrast to the situation in atherosclerosis, cleavage is driven by disintegrin and metalloproteinase domain-containing protein 9 (ADAM9) owing to downregulation of miR-126. Diabetic macrophages also have a downregulation of a proefferocytic pathway involving erythropoietin (EPO)–EPO receptor signalling. Under normal conditions, EPO receptor signalling increases PPARγ expression, leading to enhanced PPARγ-mediated transcription of a number of efferocytosis receptors and bridging molecules. In diabetic mice, however, there is a reduction in levels of both EPO and the EPO receptor that interrupts this pathway and impairs efferocytosis efficiency. MIAT, myocardial infarction-associated transcript; oxLDL, oxidized low-density lipoprotein; SPM, specialized proresolving mediator; Ub, ubiquitin.
