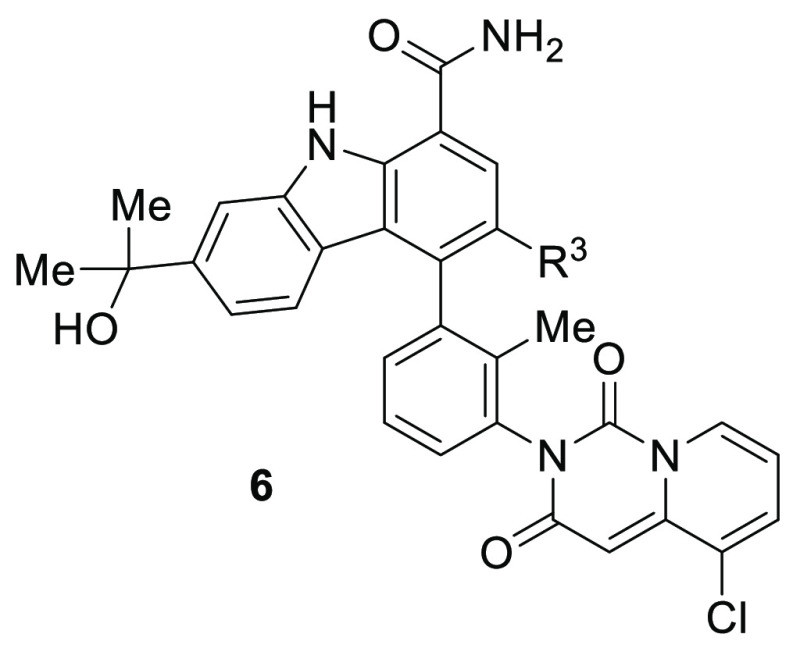
Table 2. In Vitro Potency of Carbazoles 6.
| in vitro
activity |
||||||
|---|---|---|---|---|---|---|
| compd | R3 | chirality | BTK IC50 (nM)a | JAK2/BTK selectivity | Ramos IC50 (nM)a | hWB IC50 (nM)a |
| 6a | Cl | homochiral | 180 (n = 1) | 11× | >300 | ND |
| 6b | Cl | homochiral | 0.55 ± 0.16 | 2600× | 10 ± 2 | 162 (n = 1) |
| 6c | Cl | homochiral | 17 (n = 1) | 60× | 87 (n = 1) | ND |
| 6d | Cl | homochiral | 0.26 ± 0.12 | 3800× | 6.9 ± 3.4 | 25 ± 19 |
| 6e | F | homochiral | 6.3 (n = 1) | 330× | 600 (n = 1) | ND |
| 6f | F | homochiral | 0.22 ± 0.07 | 6000× | 6.6 ± 0.9 | 64 (n = 2) |
| 6g | F | homochiral | 7.2 (n = 1) | 140× | 170 (n = 2) | ND |
| 6h | F | homochiral | 0.19 ± 0.02 | 7200× | 7.6 ± 2.4 | 37 (n = 2) |
IC50 values are shown as mean values of at least three determinations unless specified otherwise; ND = Not determined.