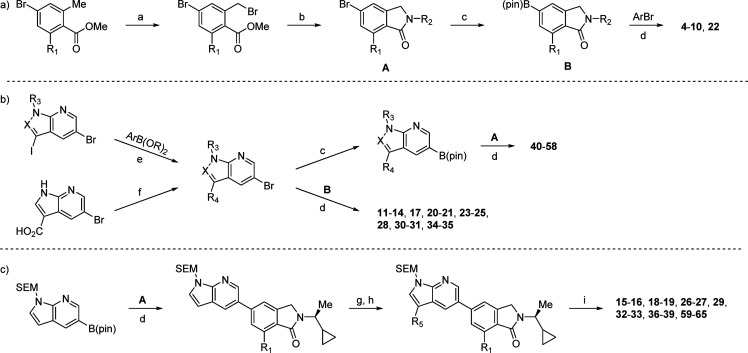
Scheme 1. General Synthetic Routes to Azaindole-Based Inhibitors.
Reagents and conditions: (a) NBS, (PhCO2)2, CCl4, 80 °C; (b) RNH2, B(OH)3, K2CO3, ACN, r.t.; (c) B2pin2, (dppf)PdCl2, KOAc, dioxane, 100 °C; (d) (dppf)PdCl2, Na2CO3, dioxane/H2O, 100 °C; then deprotection (conditions vary, see Supporting Information); (e) (dppf)PdCl2, Na2CO3, dioxane/H2O, 80 °C; (f) RNH2, HATU, i-Pr2NEt, DMF, 40 °C; (g) NBS, CH2Cl2, r.t.; (h) ArB(OR)2, (dppf)PdCl2, Na2CO3, dioxane/H2O, 100 °C; (i) TFA, r.t.; N,N-DMEDA, MeOH, 45 °C.