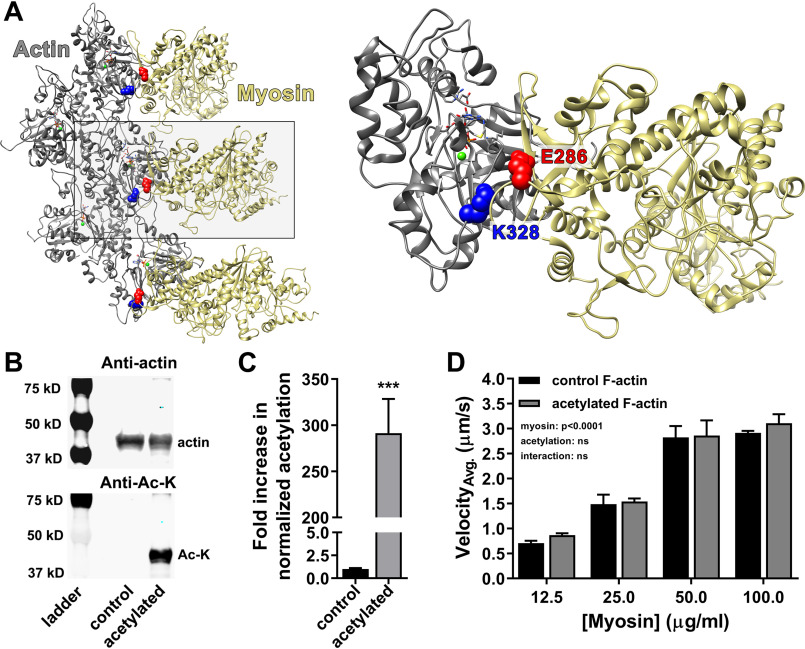
Figure 1.
Actin acetylation does not significantly alter myosin-driven F-actin sliding velocity. A, proposed Lys328–Glu286 (blue–red, respectively) actin–myosin (gray–tan, respectively) salt bridge established during strong binding (Protein Data Bank code 4A7f) (41). B, Western blots of actin treated with suprastoichiometric acetic anhydride diluted in acetonitrile (acetylated) revealed increased lysine acetylation relative to actin resuspended in acetonitrile only (control). The blots were probed with anti-actin (top panel) and anti–Ac-lysine (bottom panel; Anti-Ac-K) antibodies. C, anti–Ac-lysine intensities in B were normalized to corresponding anti-actin signals. Actin-normalized lysine acetylation increased ∼290-fold (291 ± 37) relative to control. D, in vitro motility of Alexa 568 Ph-labeled control (black) and acetylated (gray) F-actin at varying myosin concentrations. Average velocities (VelocityAvg.) were not significantly different, suggesting no change in actomyosin cross-bridge cycling caused by actin acetylation (two-way ANOVA; n = 4).