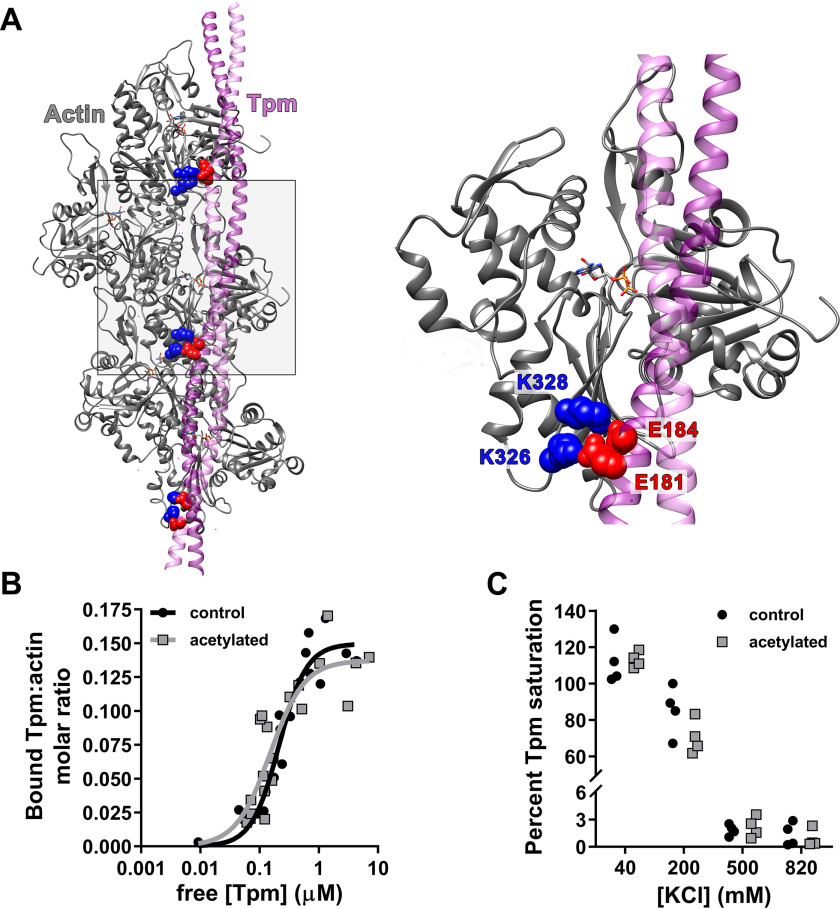
Figure 2.
Actin acetylation does not disrupt global F-actin–Tpm binding. A, purported contacts between Lys326/Lys328 (blue) on actin (gray) and negatively charged Tpm (purple) residues (red) in pseudorepeats 4, 5, and 6 (from bottom to top) (12). B, F-actin–Tpm co-sedimentation data were fit to the Hill equation (y = Bmax × xh/(Kdh + xh)), and no significant differences were found in Bmax or Kd of Tpm for control (black; Bmax = 0.15 ± 0.009; Kd = 0.21 ± 0.03 μm, respectively) versus acetylated (gray; Bmax = 0.14 ± 0.013; Kd = 0.15 ± 0.04 μm, respectively) F-actin. C, saturating amounts of Tpm were mixed with control (black) or acetylated (gray) F-actin at 40, 200, 500, and 820 mm [KCl] and pelleted. At 40 mm, the percentage of Tpm saturation of control (112 ± 6.3%) versus acetylated (113 ± 2.2%) F-actin was equivalent. Increasing [KCl] to 200 mm similarly decreased Tpm binding to control (85.4 ± 6.85%) and acetylated (70.4 ± 4.68%) F-actin, which was nearly fully ablated by 500 mm.