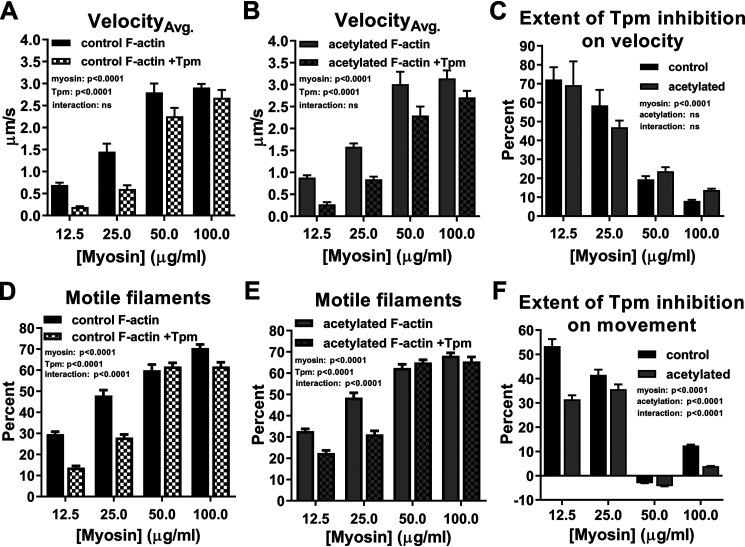
Figure 3.
Actin acetylation reduces Tpm-based inhibition of actomyosin binding. A and B, control (A, black) and acetylated (B, gray) F-actin (solid) and F-actin–Tpm (checkered) velocities significantly increased as a function of myosin concentration, whereas Tpm addition significantly decreased velocities (two-way ANOVA; p < 0.0001; n = 4). C, percentage of decrease in control (black) and acetylated (gray) F-actin velocities observed as a result of Tpm addition revealed that acetylation had no significant effect on Tpm-mediated reduction of velocity (two-way ANOVA; n = 4). D–F, the effects of myosin concentration and Tpm on percentage of moving control (D, black) and acetylated (E, gray) F-actin (solid) and F-actin–Tpm (checkered) mirrored those on velocity, whereas actin acetylation (F, gray) significantly decreased Tpm-based inhibition of filament motion relative to control (black) (two-way ANOVA; p < 0.0001; n = 17–22).