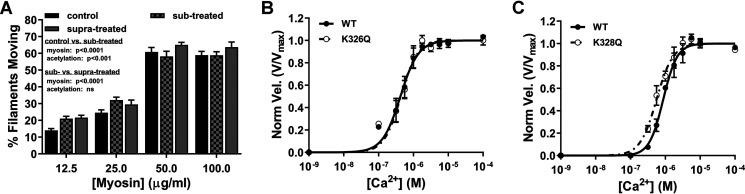
Figure 5.
Lys328 pseudoacetylation enhances RTF Ca2+ sensitivity. A, treatment of actin with a substoichiometric amount of acetic anhydride (checkered gray) significantly increased percentage of actin–Tpm filaments moving relative to control (black) (two-way ANOVA; p < 0.001; n = 9–12), whereas substoichiometric-treated F-actin–Tpm movement was not significantly different from suprastoichiometric-treated (gray) (two-way ANOVA, n = 10–12). B and C, velocity-normalized plots of Ca2+-dependent activation of K326Q- and K328Q-containing RTFs relative to respective WT controls. K326Q and K328Q maximum velocities (Vmax = 4.1 ± 0.1 and 3.3 ± 0.06 μm/s, respectively) did not significantly differ from respective internal WT controls (Vmax = 4.2 ± 0.09 and 3.3 ± 0.06 μm/s, respectively). B, Ca2+-dependent activation of K326Q-containing RTFs was equivalent to WT control actin-containing RTFs as revealed by no significant differences in Ca2+ sensitivity ([Ca2+]50 = 0.43 ± 0.029 versus 0.42 ± 0.027 μm, respectively) or cooperativity (h = 1.8 ± 0.25 versus 1.7 ± 0.21, respectively). C, although there was no significant change in cooperativity of K328Q-containing RTFs (h = 1.8 ± 0.21) relative to WT control (h = 2.2 ± 0.22), K328Q-containing RTF Ca2+ sensitivity ([Ca2+]50 = 0.56 ± 0.032 μm) was significantly increased relative to WT control ([Ca2+]50 = 0.87 ± 0.044 μm) (p < 0.0001; n = 4).