Figure 1.
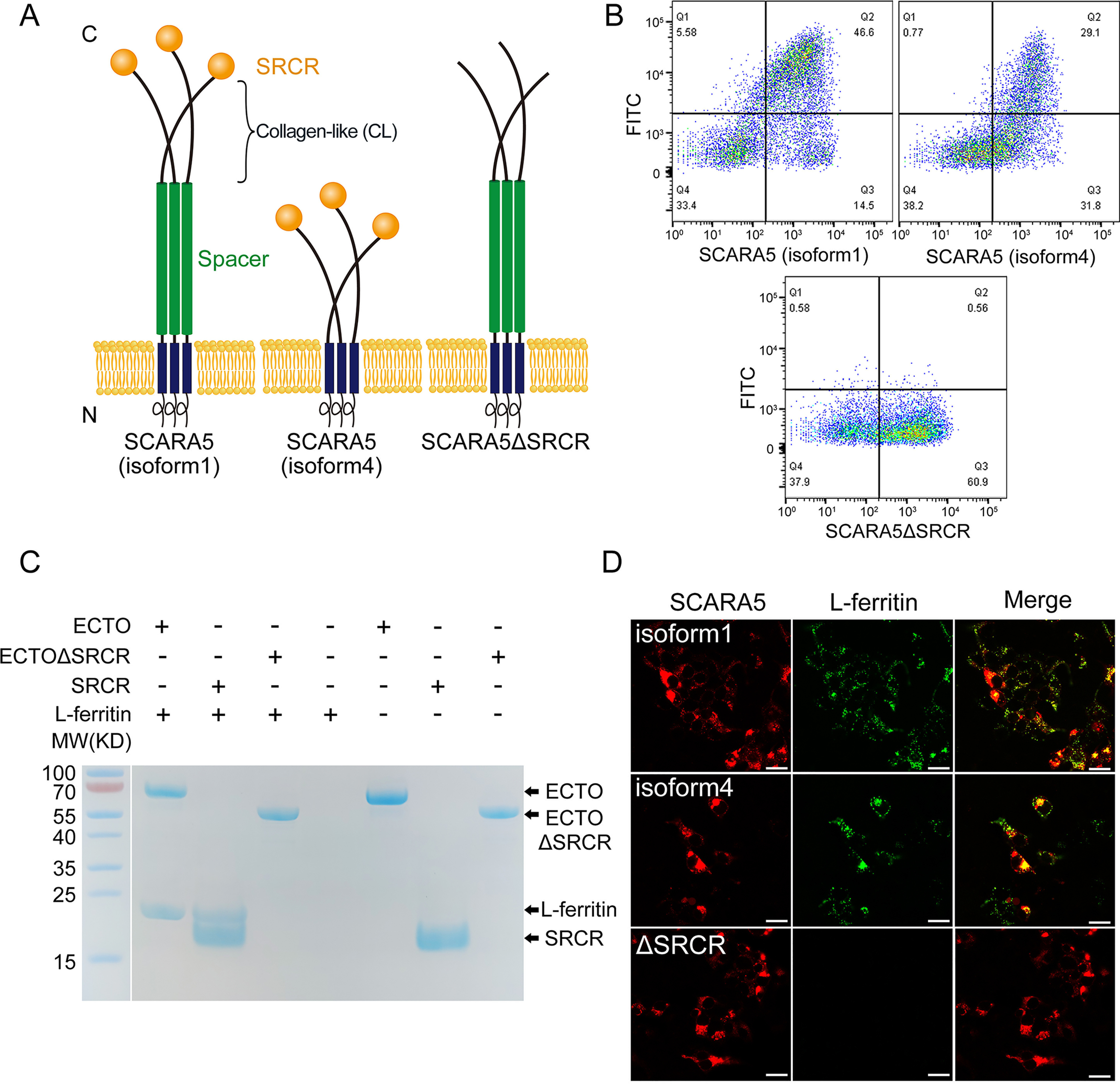
SCARA5 interacts with L-ferritin via the SRCR domain. A, schematic representation of the full-length SCARA5 (isoform 1), SCARA5 (isoform 4), and SCARA5ΔSRCR. B, FACS data showed that the FITC-labeled L-ferritin bound to the HEK293 cells transfected with hSCARA5 (isoform 1) or hSCARA5 (isoform 4) but did not bind to the SCARA5ΔSRCR-transfected cells. C, SDS-PAGE of the pulldown assays with FLAG tag showed that the ectodomain (ECTO) and the SRCR domain of SCARA5 could interact with L-ferritin, but the ectodomain without the SRCR domain (ECTOΔSRCR) had no interaction with L-ferritin. Empty beads had no interaction with L-ferritin either. D, fluorescent images showed that the hSCARA5 (isoform 1)– or the hSCARA5 (isoform 4)–transfected cells could internalize the FITC-labeled L-ferritin, but the SCARA5ΔSRCR-transfected cells had no binding to L-ferritin (bar, 25 μm).
