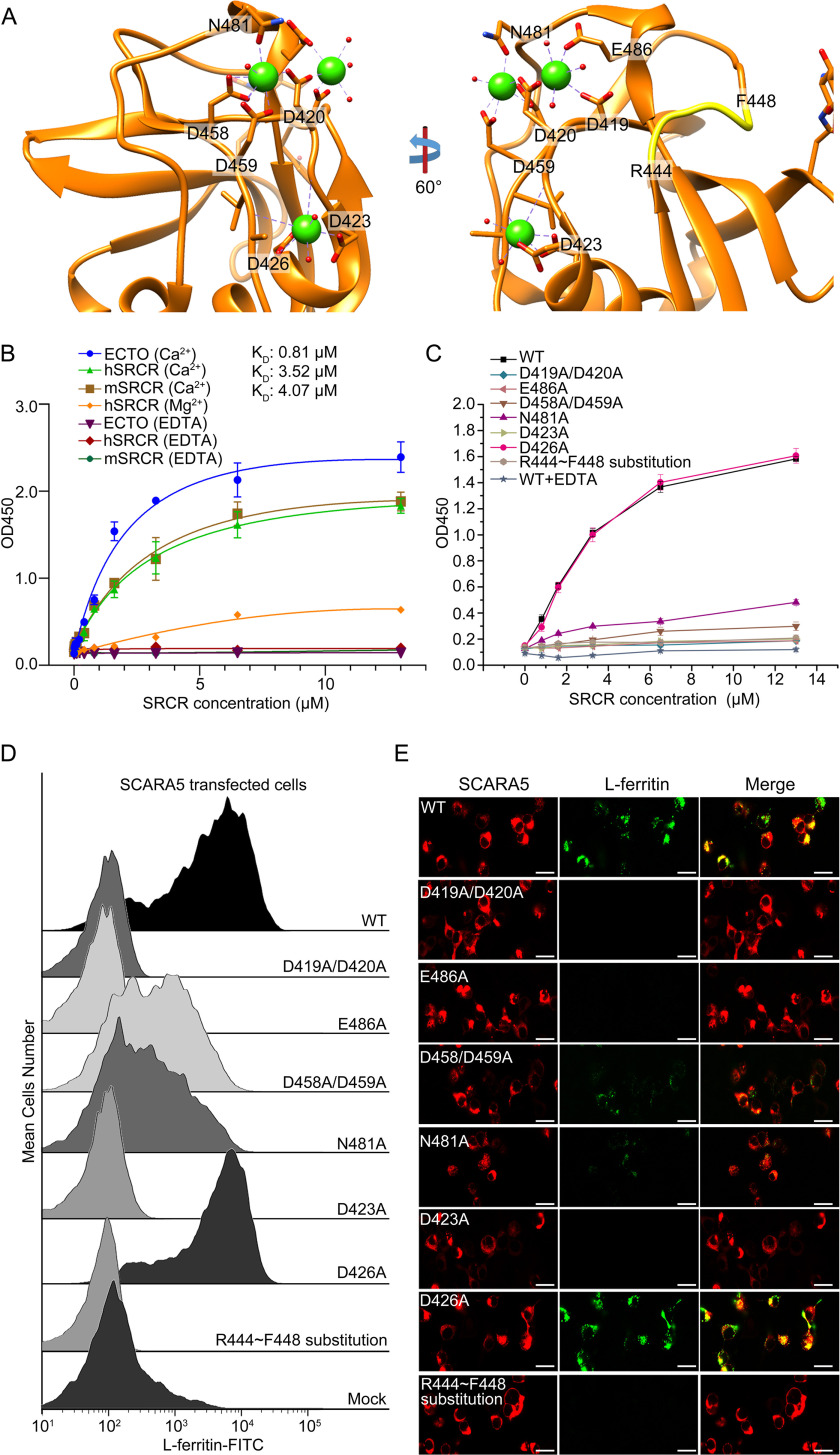
Figure 3.
Mutagenesis studies of the interaction between the SRCR domain of SCARA5 and L-ferritin. A, residues around the Ca2+-binding sites and the loop region (yellow) involved in the mutagenesis studies on the SRCR domain of hSCARA5. B, ELISA data showed that the ectodomain of hSCARA5 (ECTO) and the SRCR domains of human and mouse SCARA5 bound to L-ferritin in the presence of Ca2+ (5 mm), and the binding affinities (KD) were calculated based on the curve fitting, respectively. By contrast, Mg2+ reduced the binding affinity with L-ferritin, and EDTA could eliminate the binding completely. C, ELISA data showed that all of the mutants except D426A could reduce the binding of the SRCR domain with L-ferritin. D, FACS data showed all of the mutants of SCARA5 except D426A could reduce the binding of L-ferritin with the mutant-transfected cells. Mutants D458A/D459A and N481A from the Ca2+-binding site 2 retained some binding affinities with L-ferritin. Mock, nontransfected cells. E, fluorescent images showed that all of the mutants of SCARA5 except D426A could reduce the internalization of L-ferritin with the mutant-transfected cells (bar, 25 μm). Error bars, S.D.