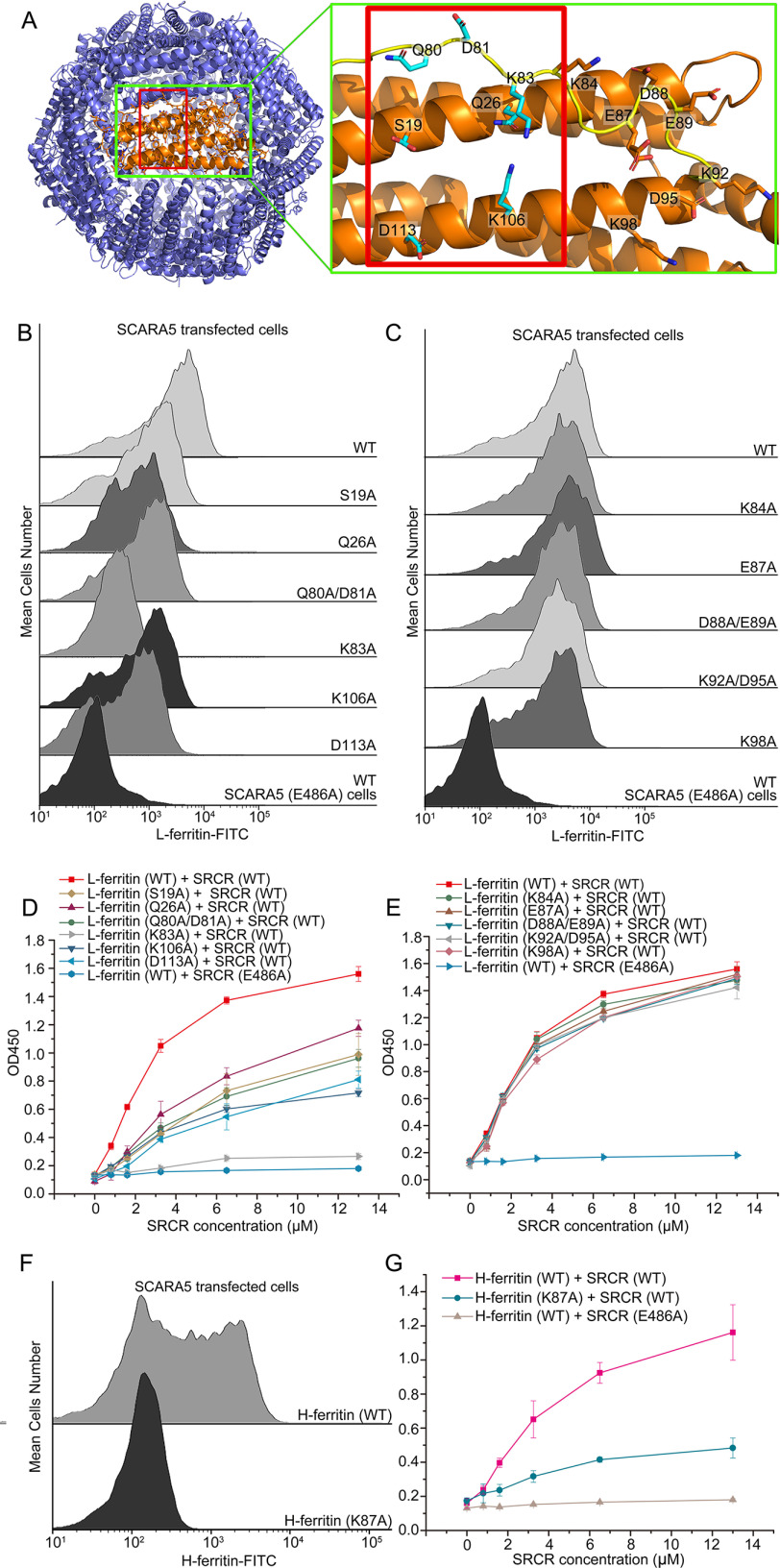
Figure 5.
Mutagenesis studies of the SCARA5 binding region on ferritin. A, residues on the surface of L-ferritin (left; Protein Data Bank entry 2FFX) involved in the mutagenesis studies are labeled in the green rectangles. The potential binding region of SCARA5 on ferritin is indicated by the red rectangles. B, FACS data showed that mutants S19A, Q26A, Q80A/D81A, K83A, K106A, and D113A of L-ferritin reduced binding affinities with SCARA5. C, FACS data showed that mutants K84A, E87A, D88A/E89A, K92A/D95A, and K98A of L-ferritin retained similar binding affinities with SCARA5 as the WT. D, ELISA data showed that mutants S19A, Q26A, Q80A/D81A, K83A, K106A, and D113A of L-ferritin reduced the binding affinities with the SRCR domain SCARA5. E, ELISA data showed that mutants K84A, E87A, D88A/E89A, K92A/D95A, and K98A of L-ferritin retained similar binding affinities with the SRCR domain SCARA5. F, FACS data showed that the mutant K87A of H-ferritin reduced the binding with SCARA5 significantly. G, ELISA data showed that the mutant K87A of H-ferritin reduced the binding with the SRCR domain of SCARA5 significantly. Error bars, S.D.