Figure 1.
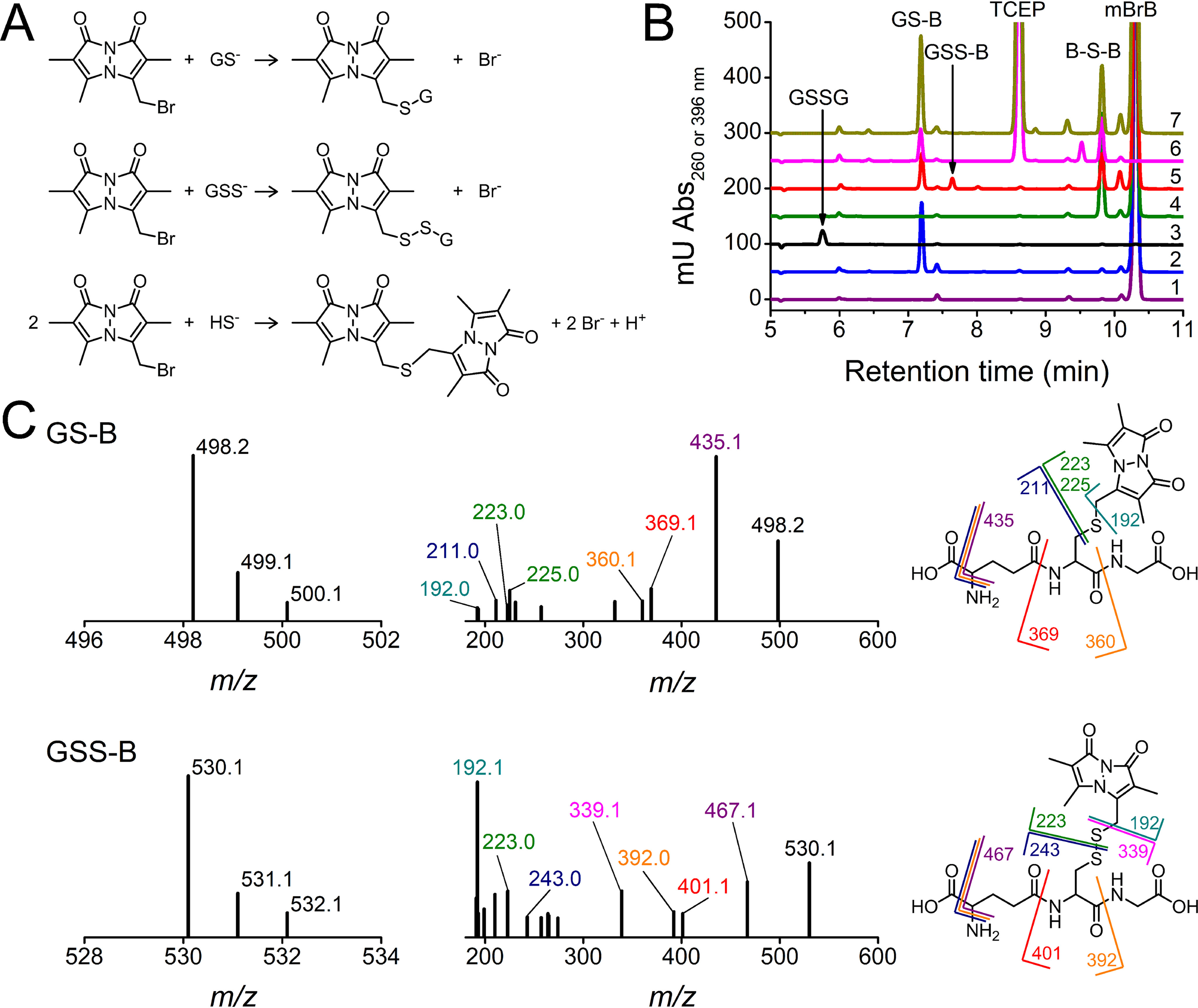
Derivatization with mBrB and characterization of chromatographic peaks. A, reactions of GSH, GSSH, and H2S with mBrB to form GS-B, GSS-B, and B-S-B, respectively. B, representative HPLC runs of 1) mBrB (2 mm), 2) GS-B (0.2 mm), 3) GSSG (1 mm), 4) B-S-B (0.2 mm), 5) mixture of GSSG and H2S (3 mm each, 20 min) diluted 15-fold and incubated with mBrB (2 mm), 6) reduction of 5 with TCEP (4.5 mm), and 7) addition of mBrB (6.9 mm) to 6. Reactions were done in phosphate buffer (0.1 m, pH 7.4, 25 °C). All chromatograms were recorded at 396 nm, except 3 (GSSG) at 260 nm. C, mass spectra and fragmentation patterns of the fractions eluting at 7.3 (GS-B, m/z 498.2) and 7.7 min (GSS-B, m/z 530.1). The product ions of GS-B are ascribable to neutral loss of NH3 and HCOOH (435.1), minus glycine (360.1), or minus a sulfur-bimane derivative (211.0), loss of glutamate (369.1), and formation of the sulfur-bimane derivative (223.0 and 225.0). The fragmentation pattern of GSS-B showed peaks analogous to GS-B plus 32 (sulfur atom), assigned to the loss of NH3 and HCOOH (467.1), minus glycine (392.0), or minus the sulfur-bimane derivative (243.0), loss of glutamate (401.1), and the formation of the sulfur-bimane derivative (223.0). The peak at m/z 339.1 could correspond to the protonated homolysis product GSS•. Fragments of m/z 192 were assigned to the bimane protonated radical as previously observed (7, 30, 36).
