Figure 3.
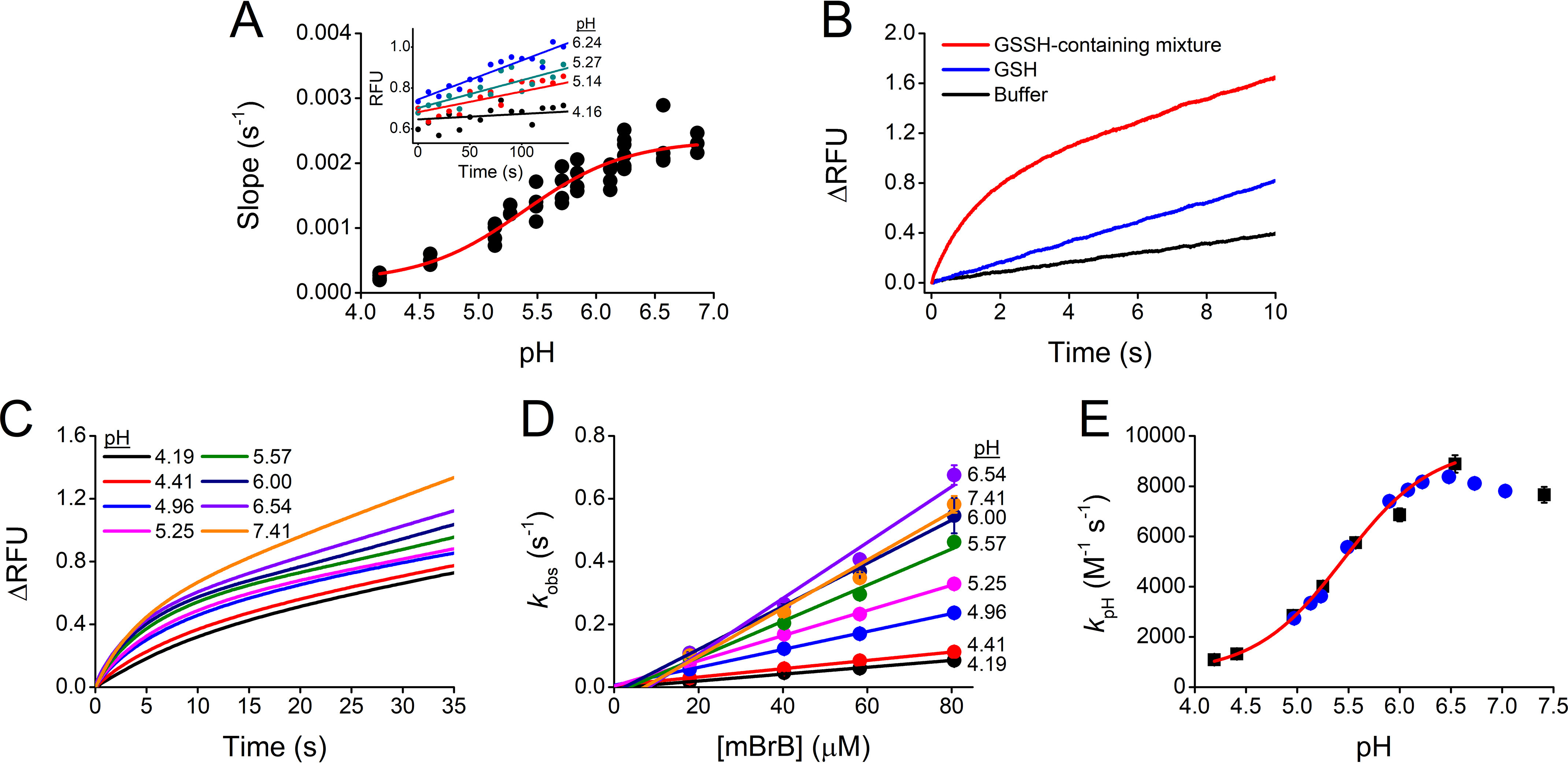
pH dependence of the reaction of GSSH with mBrB. A, a mixture containing GSSH (∼0.75 µm) reacted with mBrB (1 µm) in acetic/MES/tris buffer (pHs 4.16–6.86, 25 °C). Linear functions fitted to initial fluorescence increases (λex = 396 nm, λem = 472 nm) yielded slopes (inset) that had a sigmoid dependence with pH. The data pooled from two independent experiments gave a pKa of 5.50 ± 0.08 (parameter ± error of the fit). The points shown are quadruplicates of one representative experiment. No interference from the buffer was detected. B, representative stopped-flow fluorescence kinetic traces (λex = 396 nm, emission cutoff 435 nm) of the reaction of 118 µm mBrB with a mixture containing ∼1.6 µm GSSH, ∼6.3 µm GSH, and ∼1 µm H2S (final concentrations) in phosphate buffer (0.1 m, pH 7.40, 25 °C, 0.1 mm dtpa) (red) and with controls of 6.3 µm GSH (blue) or buffer alone (black). C, GSSH-containing mixtures (∼2 µm GSSH) reacted with 18–81 µm mBrB in acetic/MES/Tris buffer (pHs 4.19–7.41, 25 °C). Representative stopped-flow fluorescence time courses with 40 µm mBrB. Exponential plus linear or double exponential plus linear functions were fitted to kinetic traces during 10 half-lives. At the more acidic pHs, an additional fast phase was noted; the origin is unclear but its amplitude was less than 15% of the sum of the amplitudes and was not studied further. D, kobs attributed to the reaction of GSSH against mBrB concentration. The circles represent the mean ± S.D. of repetitions of a representative experiment. Error bars are usually smaller than symbols. The slopes of the fits represent the second-order rate constants. At the more alkaline pHs, a small negative y-intercept was observed, probably because of reactant impurities or to a fast reaction with a species present in low concentration. E, second-order rate constants versus pH. A one-pKa function fitted the data pooled from two independent experiments (black squares and blue circles), giving a pKa of 5.45 ± 0.03 and a maximum (pH-independent) second-order rate constant of (9.0 ± 0.2) × 103 m−1 s−1 (parameters ± errors of the fit). A small but systematic decrease in the kpH at pH > 6.5 was observed, and its cause is unknown.
