Figure 2.
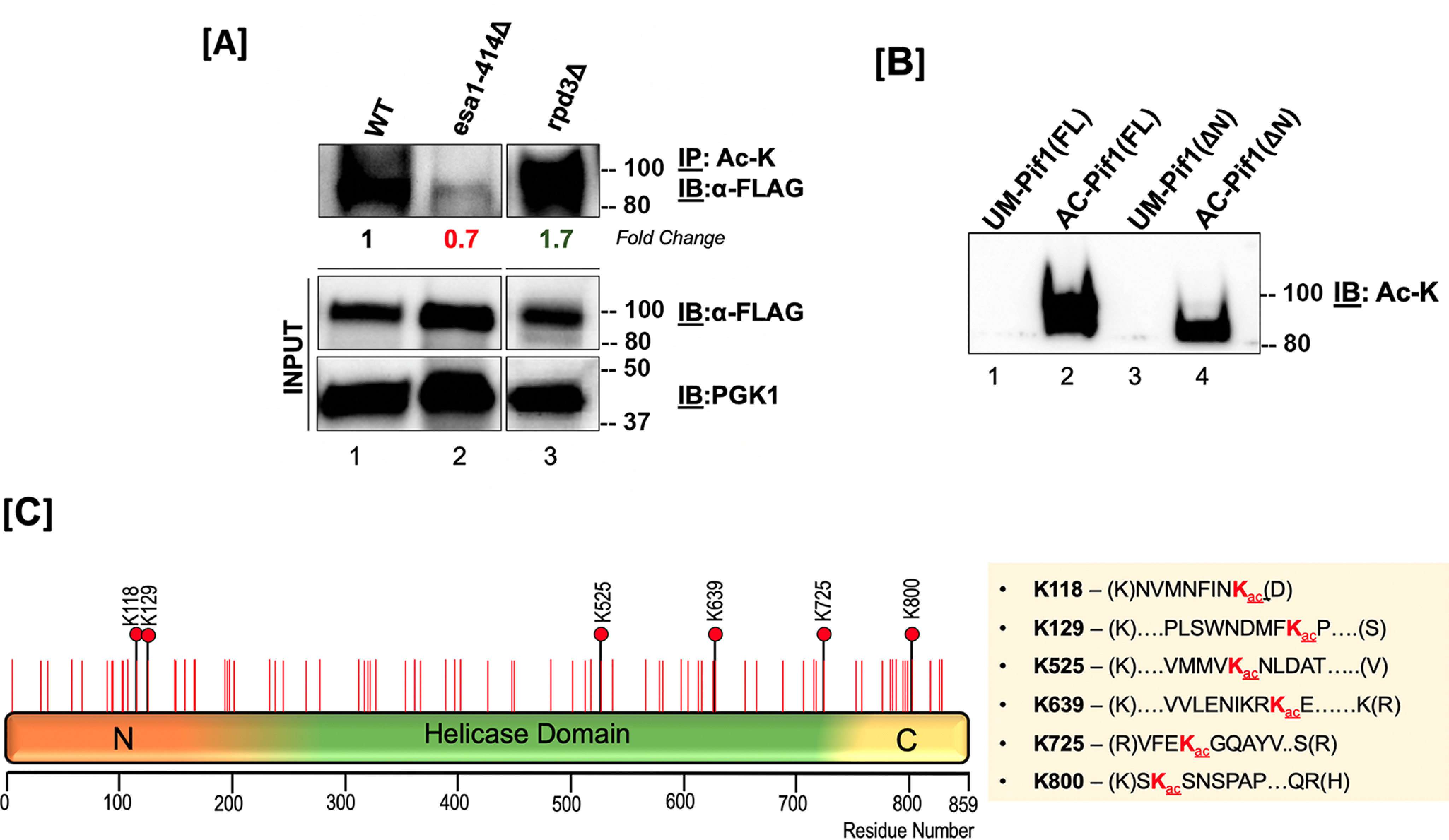
Pif1 is acetylated both in vivo and in vitro. A, top panel: S. cerevisiae lysates from WT (lane 1), esa1-414 (lane 2), and rpd3Δ (lane 3) backgrounds were immunoprecipitated with anti-acetyllysine (ac-K) antibody-coated Protein G-Dynabeads and immunoblotted with anti-FLAG antibody (1:1,000); middle panel: 10% of input immunoblotted with the anti-FLAG antibody; bottom panel: PGK-1 antibody (1:10,000). Fold-change in Pif1 acetylation is indicated. B, immunoblot of unmodified Pif1 (lane 1), NuA4 (Esa1) in vitro-acetylated full-length Pif1 (lane 2), unmodified Pif1ΔN, and NuA4 (Esa1) in vitro acetylated Pif1ΔN probed with anti-Ac-K antibody. In both A and B, molecular mass markers in kDa are denoted to the right of the blots. C, schematic of the full-length Pif1 sequence. The positions of all of the lysine residues in the sequence are denoted with red lines, and all acetylated lysine residues identified by MS are denoted with black lines and red filled circles.
