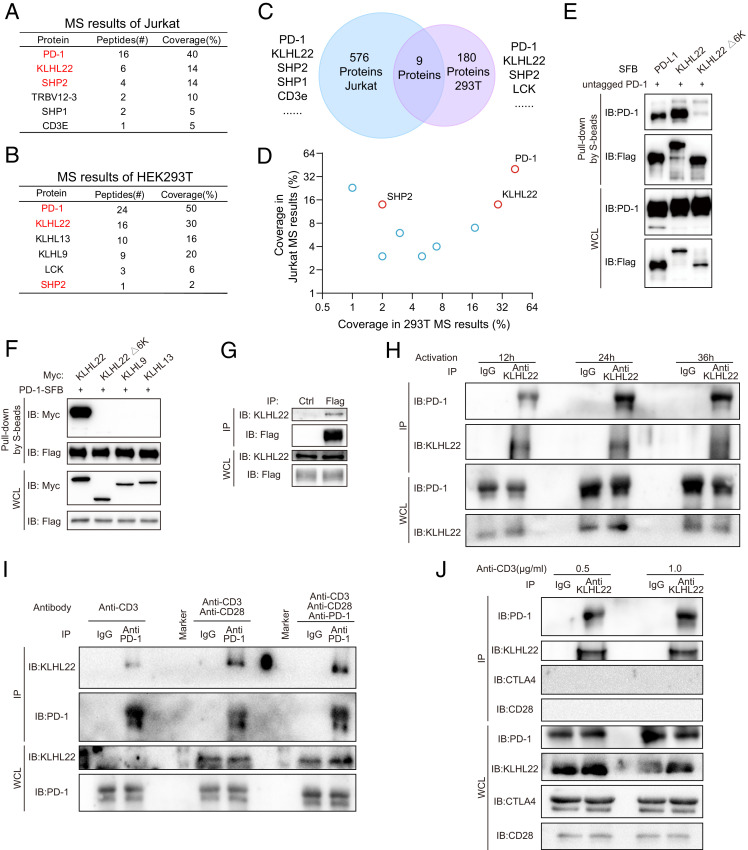
Fig. 1.
KLHL22 is a major PD-1–associated protein. (A) PD-1–associated proteins in Jurkat cells were identified by MS. Jurkat cells stably expressing PD-1–FLAG were purified with FLAG-M2 beads and analyzed by MS. Jurkat cells was stimulated by PMA (50 ng/mL) and ionomycin (1 μM) for 12 h. The table lists the selected proteins identified by MS. The full protein list is provided in Dataset S1. (B) PD-1–associated proteins in HEK293T cells were identified by TAP/MS. HEK293T cells stably expressing PD-1–SFB were purified by TAP/MS. The table lists the selected proteins identified by MS. The full protein list is provided in Dataset S2. (C and D) Venn diagram showing the overlap of two MS results. Proteins appearing in the MS results of both the Jurkat PD-1–FLAG cells and HEK293T PD-1–SFB cells. The results from the overlap shown in C are presented in a 2D graph in D. The full protein list is provided in Dataset S3. (E) 6-Kelch repeats in KLHL22 are required for the interaction between KLHL22 and PD-1. HEK293T cells were cotransfected with untagged PD-1 (PD-1 without an artificial tag) and PD-L1-SFB, SFB-KLHL22, or SFB-KLHL22Δ6K. The cell lysates were subjected to pull-down assays with S protein Sepharose and immunoblotted with the indicated antibodies. (F) HEK293T cells were cotransfected with PD-1–SFB and Myc-KLHL22, Myc-KLHL22Δ6K, Myc-KLHL9, or Myc-KLHL13. The cell lysates were subjected to pull-down assays with S-protein Sepharose and immunoblotted with the indicated antibodies. (G) Lysates of Jurkat cells stably expressing PD-1–FLAG were immunoprecipitated with FLAG-M2 beads or protein G beads with IgG and subjected to immunoblotting with the indicated antibodies. Jurkat cells were stimulated by PMA (50 ng/mL) and ionomycin (1 μM) for 12 h. (H) The endogenous interaction of PD-1 and KLHL22 in healthy human PBMCs using KLHL22 antibody pull-down. Human healthy PBMCs lysates were immunoprecipitated with an anti-KLHL22 antibody or IgG and subjected to immunoblotting with the indicated antibodies. PBMCs were stimulated by anti-CD3 (1 μg/mL) and anti-CD28 (2 μg/mL) for 12 h, 24 h, or 36 h. (I) Endogenous PD-1 associates with endogenous KLHL22 in healthy human PBMCs. Human healthy PBMCs lysates were immunoprecipitated with an anti–PD-1 antibody or IgG and subjected to immunoblotting with the indicated antibodies. PBMCs were stimulated by anti-CD3 (1 μg/mL) or anti-CD3 (1 μg/mL)/anti-CD28 (2 μg/mL) for 24 h. The third group was also treated with PD-1 antibody (2 μg/mL) for 24 h. (J) Endogenous KLHL22 associates with endogenous PD-1 in healthy human PBMCs. CD28 and CTLA4 were tested simultaneously and showed negative results. Human healthy PBMCs lysates were immunoprecipitated with an anti-KLHL22 antibody or IgG and subjected to immunoblotting with the indicated antibodies. PBMCs were stimulated by anti-CD3 (0.5 μg/mL or 1 μg/mL) and anti-CD28 (2 μg/mL) for 24 h.