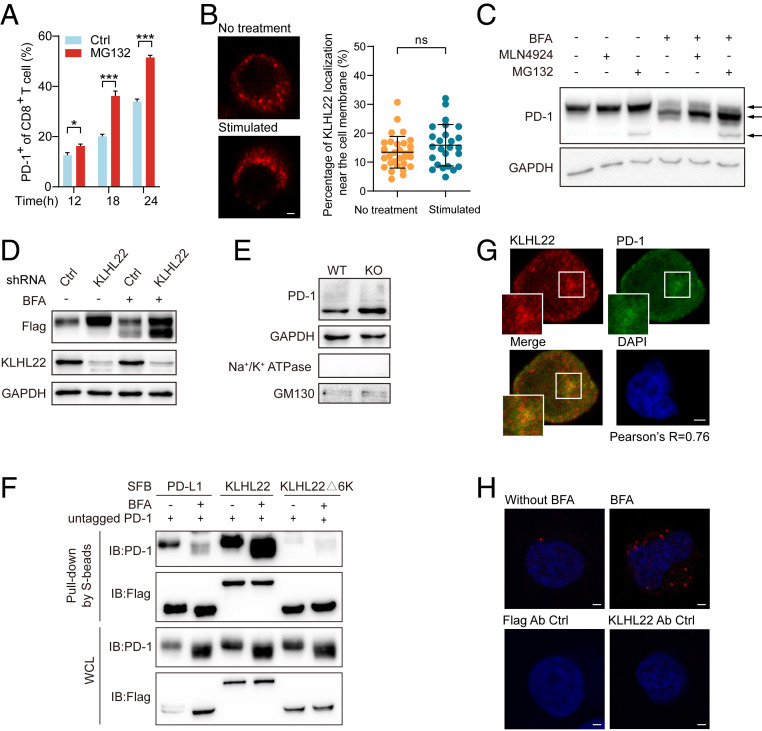
Fig. 3.
KLHL22 mediates the degradation of PD-1 before it is transported to the cell surface. (A) Cell-surface levels of PD-1 of CD8+ T cell in activated healthy human PBMCs with or without MG132 treatment were detected by flow cytometry. PBMCs were stimulated with anti-CD3 (1 μg/mL), anti-CD28 (2 μg/mL) and treated with MG132 (1 μM) for indicated hours. n = 3 repeats, *P < 0.05, ***P < 0.001, unpaired Student’s t test. (B) KLHL22 does not localize to the cell membrane in healthy human PBMCs with or without stimulation. PBMCs were incubated in the presence or absence of anti-CD3 (1 μg/mL), anti-CD28 (2 μg/mL), and subjected to immunostaining with KLHL22 antibodies. (Scale bar, 2 μm.) On the right, no significant difference in cell membrane localization of KLHL22 before and after activation. Immunofluorescence staining for KLHL22 was performed in PBMCs. MATLAB was used to identify KLHL22 near membrane location in multiple pictures and quantify the percentage of KLHL22 localization near the cell membrane. ns, not significant, unpaired Student’s t test. (C) Simultaneous treatment with MLN4924 and BFA increased the levels of incompletely glycosylated PD-1. HEK293T cells stably expressing untagged PD-1 were treated with BFA (1 µM), MLN4924 (1 µM), and MG132 (1 µM) as indicated for 12 h. The three arrows on the right side of the figure indicate fully glycosylated PD-1 (top), incompletely glycosylated PD-1 (middle), and newly synthesized PD-1 (bottom). (D) KLHL22 down-regulation leads to more accumulation of incompletely glycosylated PD-1 than fully glycosylated PD-1. The expression level of PD-1 in HEK293T cells subjected to BFA treatment and/or KLHL22 shRNA lentivirus infection was detected. HEK293T cells stably expressing PD-1–SFB were infected with lentivirus containing control or KLHL22-specific shRNA and incubated in the presence or absence of BFA (1 µM, 12 h). Cells were then subjected to immunoblotting with the indicated antibodies. (E) The amount of cytoplasmic PD-1 is higher in CD3+ T cells from Klhl22 KO mice cells than in those from WT mice. The Golgi apparatus was extracted from CD3+ T cells from WT and Klhl22 KO mice and subjected to immunoblotting to detect the protein level of PD-1 in the Golgi apparatus. Na+/K+ ATPase served as a cell membrane marker, whereas GM130 served as a Golgi apparatus marker. (F) KLHL22 has a higher affinity for incompletely glycosylated PD-1 than for fully glycosylated PD-1. HEK293T cells stably expressing untagged PD-1 were transfected with the indicated plasmids in the presence or absence of BFA (1 µM, 12 h), and cell lysates were subjected to pull-down assays with S-protein Sepharose and immunoblotted with the indicated antibodies. (G) PD-1 colocalizes with KLHL22 in the cytoplasm in Jurkat cells. Colocalization of PD-1 and endogenous KLHL22 in Jurkat cells stably expressing PD-1–FLAG was confirmed by immunostaining with anti-KLHL22 and anti-FLAG antibodies. Jurkat cells were stimulated with PMA (50 ng/mL 12 h)/ionomycin (1 µM 12 h) and treated with BFA (1 µM, 6 h). Pearson’s r = 0.76. (Scale bar, 2 μm.) (H) PLA was used to detect the colocalization of PD-1 and endogenous KLHL22 in Jurkat PD-1–FLAG stable cell lines in the presence or absence of BFA (1 µM, 6 h). Using of only one antibody (anti-KLHL22 or anti-FLAG) served as the negative control groups. (Scale bar, 2 μm.)