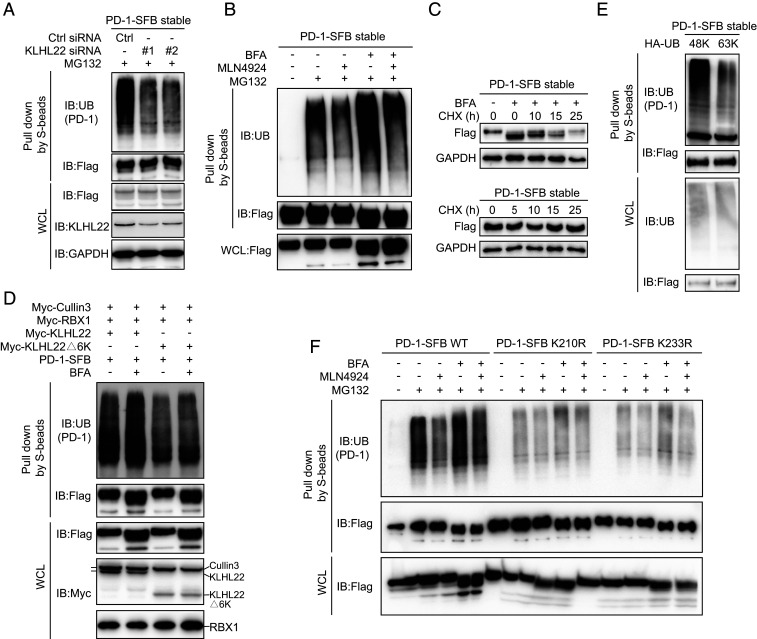
Fig. 4.
KLHL22 mediates polyubiquitination-directed degradation of incompletely glycosylated PD-1. (A) PD-1 ubiquitination is inhibited by KLHL22 depletion. Control or KLHL22-specific siRNA was transfected into HEK293T cells stably expressing PD-1–SFB in the presence of MG132 (1 µM 24 h). Cell lysates were subjected to pull-down assays by S-protein Sepharose and immunoblotted with the indicated antibodies. (B) PD-1 ubiquitination is inhibited upon MLN4924 treatment. HEK293T cells stably expressing PD-1–SFB were treated with BFA (1 µM), MLN4924 (1 µM), and MG132 (1 µM) as indicated for 12 h, and cell lysates were subjected to pull-down assays with S-protein Sepharose and immunoblotted with anti-FLAG and antiubiquitin antibodies. (C) Incompletely glycosylated PD-1 is unstable in vivo. PD-1–SFB stable cells were incubated in medium containing 10 μg/mL cycloheximide (CHX) in the presence or absence of BFA (1 µM) for the indicated time. Western blotting was carried out using the indicated antibodies. (D) The KLHL22/CUL3/RBX1 complex ubiquitinates PD-1 in vivo. CUL3, RBX1, and either KLHL22 or KLHL22Δ6K were overexpressed in HEK293T cells stably expressing PD-1–SFB, and cell lysates were subjected to pull-down assays with S-protein Sepharose and immunoblotted with the indicated antibodies. (E) Only 48K ubiquitin can be conjugated to PD-1. For ubiquitination mutants transfected into HEK293T cells stably expressing PD-1–SFB, all lysine’s were mutated to arginine except Lys48 (48K) or Lys63 (63K). Cells were treated with MG132 (1 µM, 24 h). Ubiquitination of PD-1 was detected by immunoblotting with antiubiquitin antibody. Cell lysates were subjected to pull-down assays with S-protein Sepharose and immunoblotted with antiubiquitin and anti-FLAG antibodies. (F) The ubiquitination of PD-1 on K210R and K233R is significantly reduced. PD-1–SFB (WT), PD-1–SFB (K210R), or PD-1–SFB (K233R) was transfected into HEK293T cells treated with BFA (1 µM), MLN4924 (1 µM) and MG132 (1 µM) as indicated for 12 h. The resulting cell lysates were subjected to pull-down assays with S-protein Sepharose and immunoblotted with the indicated antibodies.