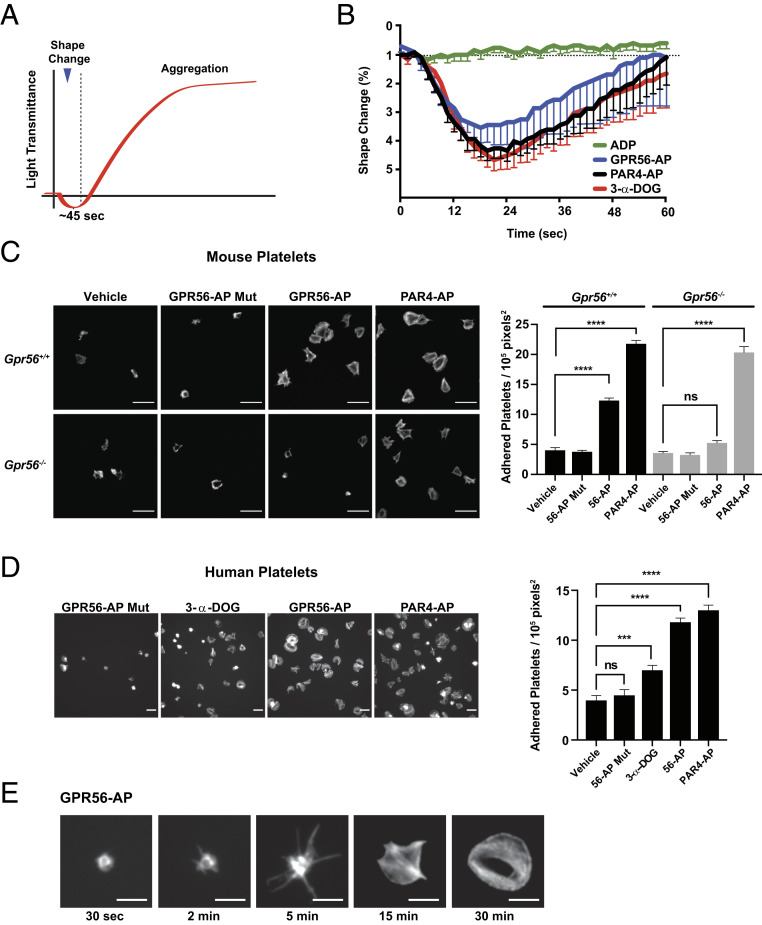
Fig. 4.
GPR56 induces platelet shape change, filopodia extension, and spreading. (A) Schematic showing idealized light transmittance phases of shape change and aggregation of agonist-treated platelet suspensions. (B) GPR56 agonists (GPR56-AP and 3-α-DOG) and PAR4-AP induce platelet shape change in the presence of integrin and purinergic receptor inhibitors (RGDS synthetic peptide and 2-MeSAMP). Values are mean ± SEM. (C) Gpr56+/+ and Gpr56−/− mouse platelet spreading onto vitronectin-coated glass in response to GPR56-AP, GPR56-AP mutant, and PAR4-AP. Shown are representative fields of phalloidin-stained platelets at 100× magnification. Quantification is adhered platelets per unit area (105 pixels2, n = 15). Values are mean ± SEM. One-way ANOVA. ****P ≤ 0.0001. (Scale bar, 10 µm.) (D) Human platelet spreading onto vitronectin-coated glass in response to GPR56-AP, GPR56-AP mutant, 3-α-DOG, and PAR4-AP. Shown are representative fields of phalloidin-stained platelets at 100× magnification. Quantification is adhered platelets per unit area (105 pixels2, n = 15). Values are mean ± SEM. One-way ANOVA. ***P ≤ 0.001; ****P ≤ 0.0001. (Scale bar, 10 µm.) (E) Time course of human platelet spreading onto vitronectin-coated glass in response to GPR56-AP. Representative images of phalloidin-stained platelets are from n = 4 experiments and show progressive filopodia protrusion, lamellipodia formation, and spreading. (Scale bar, 5 μm.) ns, nonsignificant.