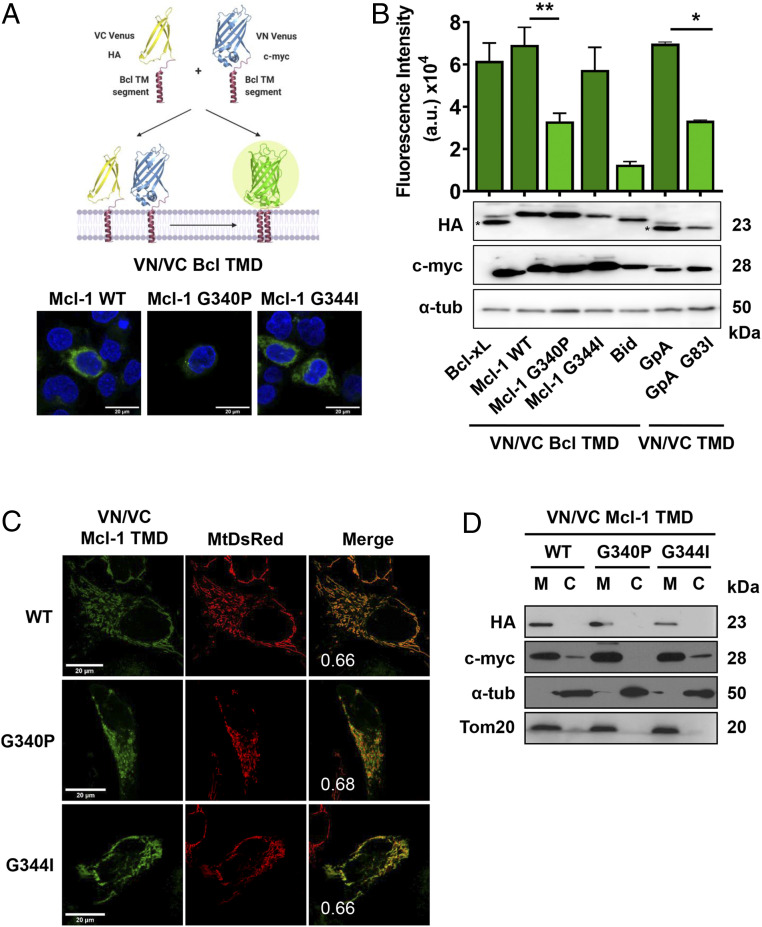
Fig. 1.
Mcl-1 TMD homooligomerizes in the mitochondrial membrane. (A) Venus reconstitution depends on interactions established between Bcl TMDs. Self-association of the wild-type Mcl-1 TMD and single amino acid substitution variants measured by BiFC in HCT116 cells. Representative images of fluorescent signal in the confocal microscope are shown. Blue signal corresponds to Hoescht staining and green signal to Venus reconstitution. The Bcl-xL TMD and the GpA TMD homooligomerization served as positive controls and the GpA G83I and Bid TMD as negative controls. Asterisk (*) indicates predicted size. (B) Graphical representation of the Venus fluorescent quantification by spectrophotometer. Error bars represent the mean ± SEM, n = 6. Significant differences compared to the Mcl-1 WT TMD analyzed using Dunnett’s multiple comparison test (95% CI). *P < 0.05, **P < 0.01. Chimeric protein expression of VN (c-myc) and VC (HA) constructs compared in the Bottom; α-tubulin used as a loading control. Asterisks indicate the mobility expected for Bcl-xL and Bid constructs. (C) Confocal images of HeLa cells expressing MtDsRed marker and transfected with VN and VC Mcl-1 TMD constructs. Homooligomers formation was observed in the green channel. Colocalizations are shown in yellow with the PCC. (D) Subcellular fractionation of HCT116 cells transfected with VN (c-myc) and VC (HA) TMD constructs was controlled using Tom20 (mitochondrial fraction, M) and α-tubulin (cytosol, C).