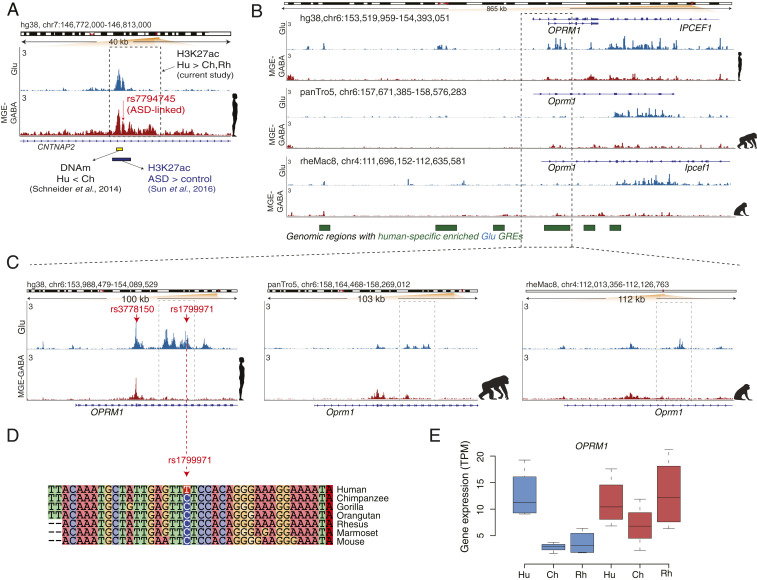
Fig. 4.
Human-specific up-regulated GREs harbor genes implicated in language, ASD, and opioid addiction. (A) Evolutionary regulatory (H3K27ac and DNA methylation) and ASD-associated changes at an enhancer within the CNTNAP2 locus in humans (also see SI Appendix, Fig. S4B). The enhancer (shown in a dashed box) is located within the second intron of CNTNAP2 and shows human-specific up-regulation of the H3K27ac signal in MGE-GABA neurons. Shown are: ASD-associated SNP rs7794745 (red arrow) (59), the region with the largest decrease in DNA methylation (DNAm) in Hu vs. Ch (green bar) (53), and the position of an H3K27ac peak (brown bar) that is up-regulated in ASD vs. control subjects (55). (B) The OPRM1 locus shows a high density of Glu human-specific up-DA GREs (the regions marked as green boxes depict areas with one or several Hu up-DA GREs). The leftmost region exemplifies an evolutionary respecification change from a Rh-enriched enhancer in MGE-GABA to a Hu-enriched enhancer in Glu. (C) H3K27ac profiles within the OPRM1 locus in three species and two neuronal subtypes. Evolutionary regulatory changes were found within the 5′ region, including human up-regulated promoter and enhancer GREs. The opioid abuse-associated SNPs rs3778150 and rs1799971 (red arrows) overlap with a human-specific up-DA promoter and human-specific up-DA enhancer, respectively. (D) Genomic alignment of the 40-bp region centered on the rs1799971 SNP in humans. The human-specific nucleotide substitution at the SNP position (C → T) is highlighted. Notice a high level of sequence conservation in the immediate vicinity of the SNP. The C nucleotide in humans represents the minor allele, which has been associated with opioid abuse (65). (E) Evolutionary changes in gene expression of OPRM1 in Glu neurons. Gene-expression changes were concordant with the regulatory changes, suggesting human-specific and Glu-specific up-regulation of OPRM1.