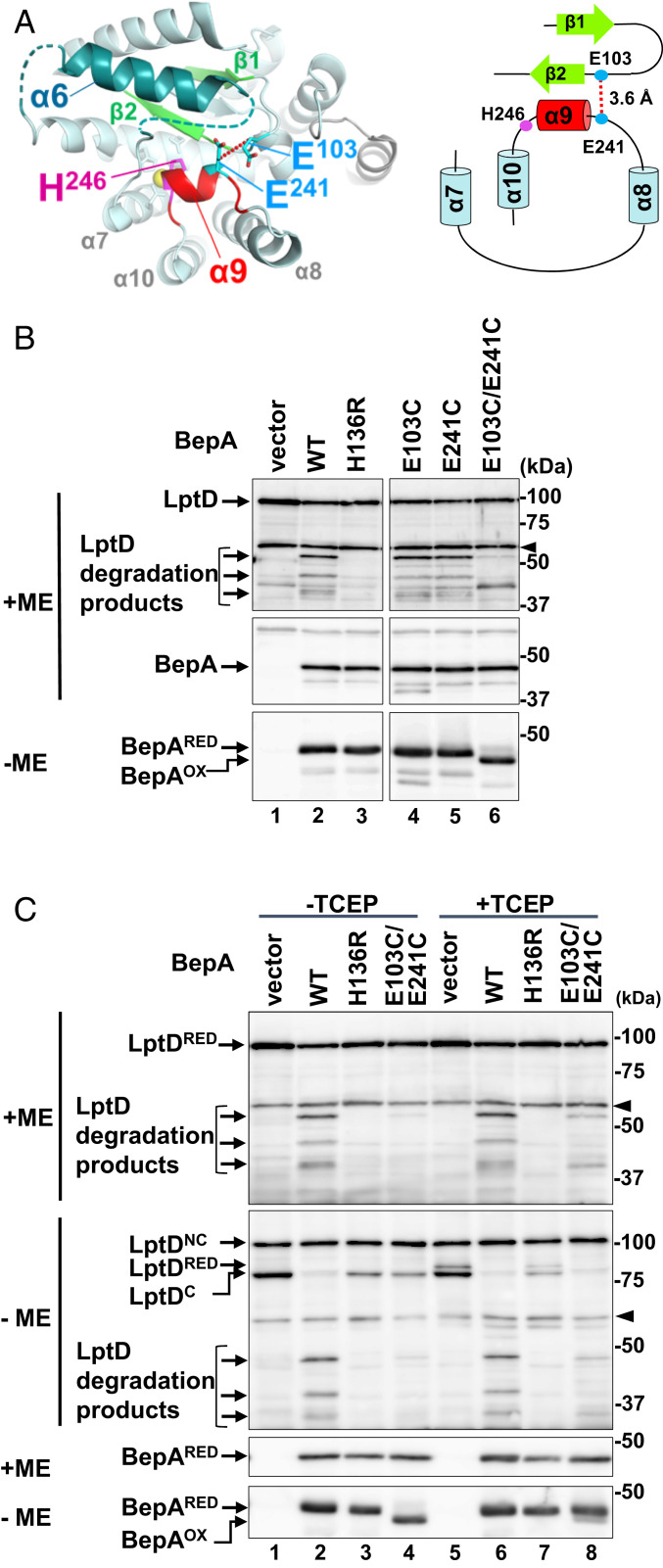
Fig. 3.
Tethering of the helix α9 inhibits degradation of overproduced LptD by BepA. (A) A close-up view and a schematic representation of the regions connected by the disulfide-bond formation between E103C and E241C (shown in cyan). The distance between β-carbons of Glu-103 and Glu-241 is shown. (B) LptD degradation by the α9/H246 loop-fixed mutants of BepA. ΔbepA/pTWV-lptD-His10 cells carrying an empty vector pSTD689 (vector) or either of the plasmids encoding the indicated BepA derivatives were grown in M9-based medium with 1 mM IPTG, and total cellular proteins were analyzed by reducing (+ME) or nonreducing (−ME) SDS/PAGE and immunoblotting with anti-LptD (Upper) or anti-BepA (Middle and Lower) antiserum. An ∼40-kDa band reacted with LptD antiserum in lanes for vector (lane 1) and E103C/E241C (lane 6) samples is an unknown background that was detected occasionally. The arrowheads indicate nonspecific bands serving as a loading control. (C) Effects of disulfide cleavage by TCEP on the LptD degradation by the E103C/E241C mutant. Cells were grown and analyzed as in B except that they were treated with 1 mM (final concentration) TCEP for 10 min at an early log phase before sampling. The representative results of two independent replicates are shown. BepAOX and BepARED indicate the disulfide-oxidized and reduced form of BepA(E103C/E241C), respectively.