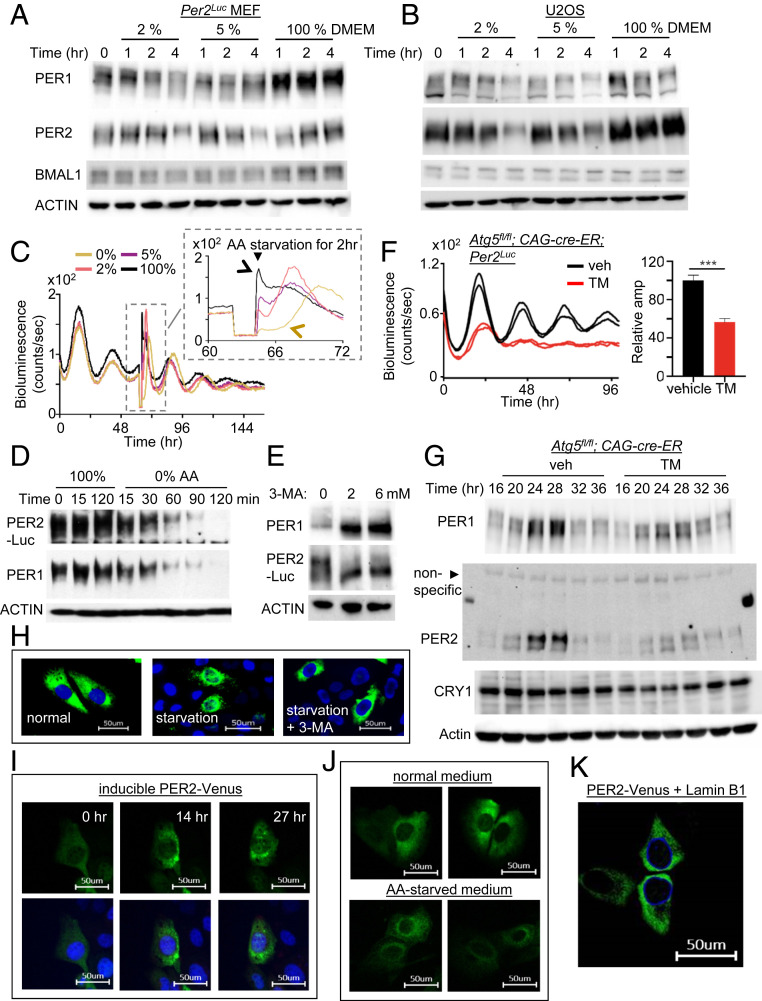
Fig. 2.
PER molecular rhythms are also modulated by autophagy-enhancing and inhibiting conditions. (A and B) PER rhythms were accelerated after MEFs and U2OS were subjected to starvation. Unsynchronized cells were subjected to starvation and harvested at indicated times. PER phosphorylation is reflected by slower electrophoretic mobility, as established previously (10). Note that PER phosphorylation and breakdown were accelerated by the starvation. (C) A pulse of starvation induced a rapid drop in bioluminescence in a dose-dependent manner. Note that the new phases after 100% and 0% AA pulses are indicated by black and brown arrows, respectively. All four samples show baseline signals during the 2-h pulse in the Inset because the samples were removed from the LumiCycle for the pulse. Note that the phase of 0% (brown) seems delayed relative to that of the control (black) in the Inset, but the 0% (brown) peak is from the next cycle relative to that of the control (black) peak (see D). However, it is less clear for the 2% and 5% cases, because the current cycle during the pulse was partially affected and could be recovering after the pulse, which will result in delays. In any case, our data show that pulses of starvation can induce stable phase shifts through modulating the pacemaker PER. (D) PER phosphorylation and breakdown were dramatically accelerated by starvation with 0% AA. Note that the current cycle of PER ended within 2 h for the 0% sample. (E) Treatment with autophagy inhibitor 3-MA induced accumulation of hypophosphorylated PER. (F and G) Homozygous Atg5 null mutant MEFs combined with the endogenous Per2Luc reporter (18) showed dramatically dampened bioluminescence and molecular rhythms. Period could not be determined from the Atg5 mutant cells due to the low amplitude. n = 3 each. Data are representative of two experiments. (H) Nuclear accumulation of PER2-Venus was accelerated by starvation (5% AA) in U2OS, but this was abolished by 3-MA treatment. (I) Nuclear entry of PER2-Venus was gated. Note that PER2-Venus is highly enriched in the perinuclear area. The same cell was monitored for 27 h after discontinuation of PER2-Venus induction (Movie S1). Nuclear entry was not complete even after 27 h, because endogenous CK1δ/ε were limiting to the transgenic PER2-Venus (SI Appendix, Fig. S5) (Scale bar: 50 μm.) (J) Perinuclear enrichment of PER2-Venus was more pronounced by starvation. U2OS cells were incubated with normal or 5% AA medium for 12 h after discontinuation of PER2-Venus induction (Scale bar: 50 μm.) (K) Perinuclear localization of PER2-Venus was confirmed by coexpressing a nuclear membrane protein, Lamin B1. Lamin B1 was expressed as an mCerulean3 fluorescent protein.