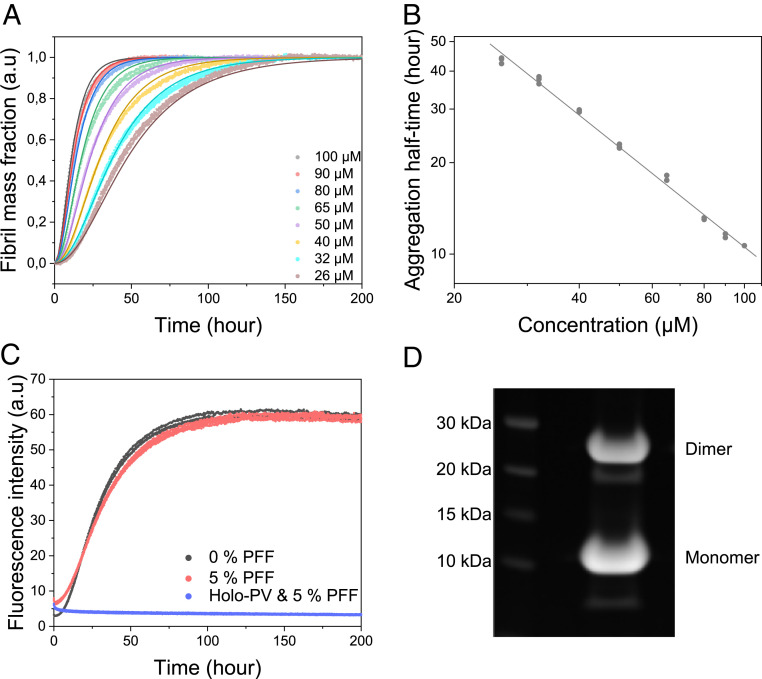
Fig. 1.
(A) Aggregation kinetics of apo β-PV as a function of monomer concentration, as measured by ThT fluorescence at 37 °C under quiescent conditions (150 mM NaCl, 1 mM CaCl2, 5 mM EDTA, and 25 mM Tris⋅HCl pH 7.4). Solid lines indicate fits to data based on a primary nucleation model in the AmyloFit web interface (7) (SI Appendix, Fig. S2B, nonnormalized data). (B) Double-logarithmic plot of amyloid formation half-time vs. apo β-PV concentration extracted from the ThT data; the fitted line has a slope (γ) of −1.1. (C) Aggregation kinetics of 50 µM apo β-PV in the presence of 5% monomer equivalent preformed apo β-PV amyloid fibrils (PFFs) at the same conditions as in A. (D) SDS/PAGE of apo β-PV fibrils obtained after incubation of protein monomers at 50 µM for 300 h.