FIGURE 1.
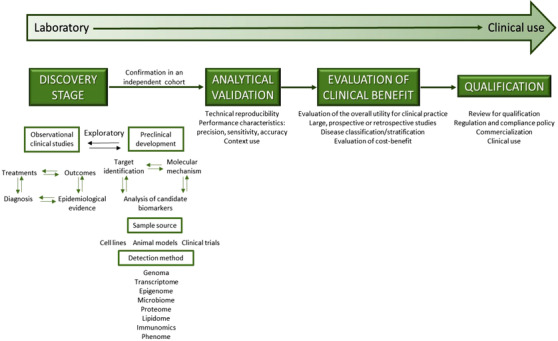
Biomarker development process from bench to bedside. Basic research in cancer biology provides preclinical data and new technology to support clinical advances in biomarkers discovery related to early detection (to determine specific healthy disorder), prognosis (to chart the likely course of the disease), prediction (to indicate drug response), and predisposition (to indicate risk of developing disease). Initially, clinical translational research had two main phases: (1) bench‐to‐bedside, in which new discoveries from the laboratory could be translated toward clinical research (proof of concept, phases I and II clinical trials) and (2) clinical use, in which these applications could be translated into the practice (phase III clinical trials, studies of clinical efficacy and development of guidelines). Recently, this process was further expanded by the addition of four more elements: discovery stage, analytical validation, evaluation of clinical benefits, and qualification
