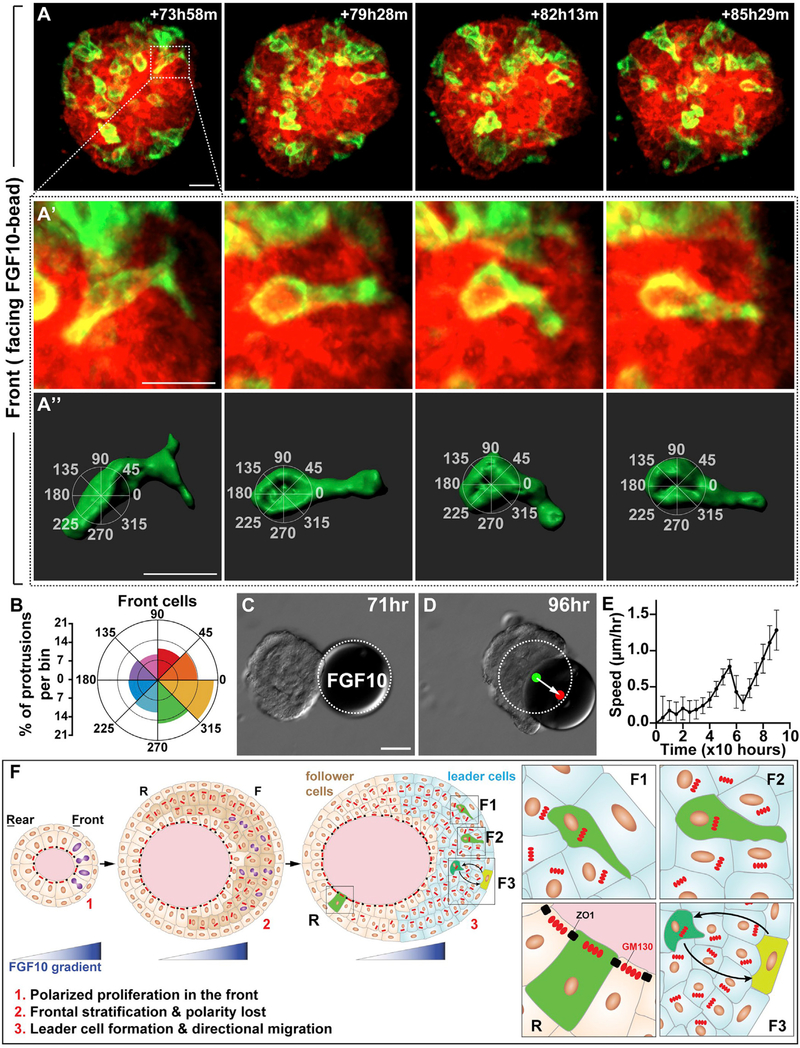
Figure 7. Leader Cell Protrusions Are Oriented toward the FGF10 Source.
(A–A″) A representative 3D reconstruction of a front cell with protrusions, imaged during collective migration induced by FGF10. (A″) Overlaying method for assigning protrusions to 45° bins, with the 0–180° axis aligned with the migration direction. Scale bars, 20 μm.
(B) Protrusions per bin as quantified from front cells of the organoid. Two-sample Hotelling᾽s T-squared test showed a significant mean direction (p < 0.01). A total of 1,575 protrusions from 25 cells of 17 organoids were used for this analysis. See Method Details.
(C and D) Still DIC images of organoid during migration at the times indicated. Note that the FGF10 bead was pushed by the migrating organoid. White dotted line denotes the original position of the FGF10 bead. Green and red dots indicate the center of gravity of the bead at the beginning and end, respectively. Arrow indicates the distance and direction of bead displacement. Scale bars, 100 μm.
(E) Organoid speed was measured every 4 h, starting with the bead-contacted organoid (n = 5). Note that organoid speed briefly slowed when it touched the bead before it accelerated again.
(F) A model of the FGF10 function during directional collective migration of the mammary gland epithelium. The FGF10 gradient promotes preferential cell proliferation (cell with purple nucleus) in the organoid front and thus sets up the “front-rear” polarity (1). As a result, the organoid front becomes stratified (dark beige cells) and loses tissue polarity (inner layer cells with GM130 irregular arrangement and loss of tight junction) (2). The FGF10 also orients intra-epithelial protrusions toward the signal source (F1 and F2) and coordinates movements (F3) of individual front leader cells (3). As a result, leader cells drive mammary organoids to undergo directional migration. Note that cell protrusions in the rear “passenger” cells are random (R). Moreover, cells lining the lumen are unlikely to be driver cells because they still show tissue polarity, as marked by the expression patterns of ZO1 and GM130 typically observed in the polarized epithelium.