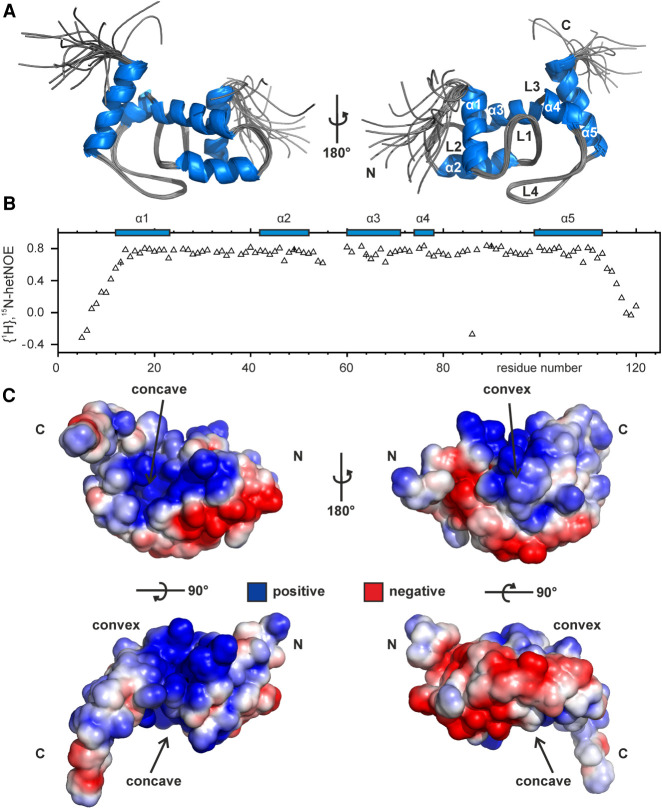
FIGURE 2.
NMR solution structure and electrostatic surface features of Lpp1663. (A) Lpp1663 has a ProQ/FinO fold with five central helices that are connected by four structurally well-defined loops. Shown is an overlay of the 10 final structures with an rmsd of 0.4 Å (ordered residues) that were deposited in the PDB under accession number 6S10. (B) The {1H}, 15N heteronuclear NOE of 15N-labeled Lpp1663 indicates the flexible amino- and carboxyl termini of the protein. Secondary structure elements are depicted by blue boxes. (C) The electrostatic surface potential of the lowest energy structure reveals two positively charged patches on either side of the protein, the concave and the convex site. Positive and negative charges are colored in blue and red. Amino- and carboxyl termini are indicated as N and C, respectively.