Fig. 1. PKC family kinases phosphorylate the EphA2 S892 motif in vitro.
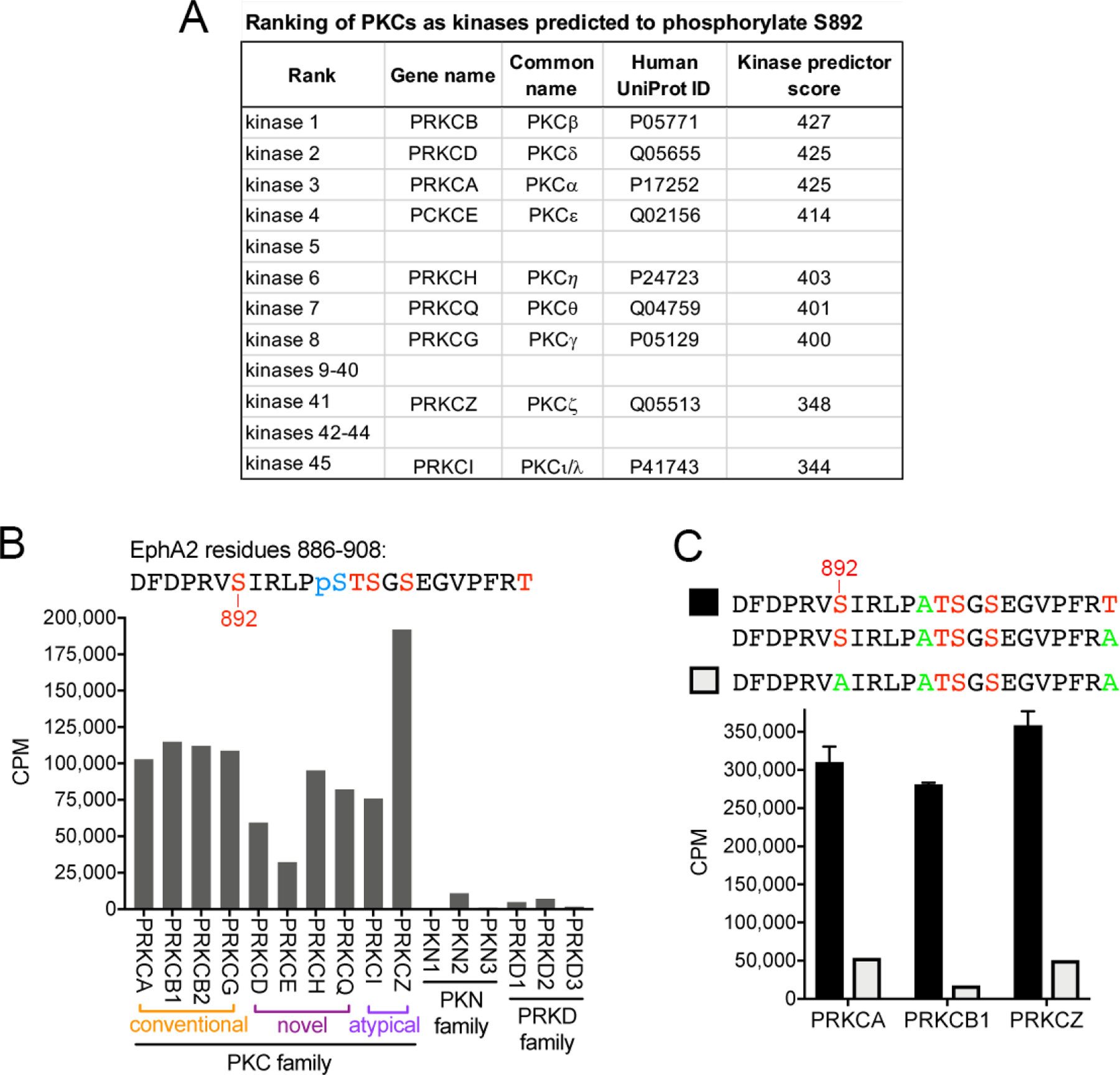
(A) Ranking of the nine PKC family members among the kinases predicted to phosphorylate the EphA2 S892 motif, based on the PhosphoNET kinase predictor score. (B) In vitro kinase reactions with radioactive ATP show that most PKC kinases (including PKCβ isoforms 1 and 2) phosphorylate at high levels a peptide substrate in which S892, T898, S899, S901 and T908 are potential phosphorylation sites (indicated in red). Phosphorylated S897 (pS897, blue) was incorporated during peptide synthesis, preventing incorporation of radioactive phosphate at this site during the in vitro kinase reaction. Other closely related kinases (PKN and PRKD families) do not or only minimally phosphorylate the peptide. CPM, counts per minute. (C) Three representative members of the PKC family phosphorylate peptide substrates containing S892, whereas phosphorylation is greatly reduced when S892 is mutated to Ala (green), which cannot be phosphorylated. This demonstrates that PKC kinases preferentially phosphorylate the S892 motif in the EphA2 kinase-SAM linker.
