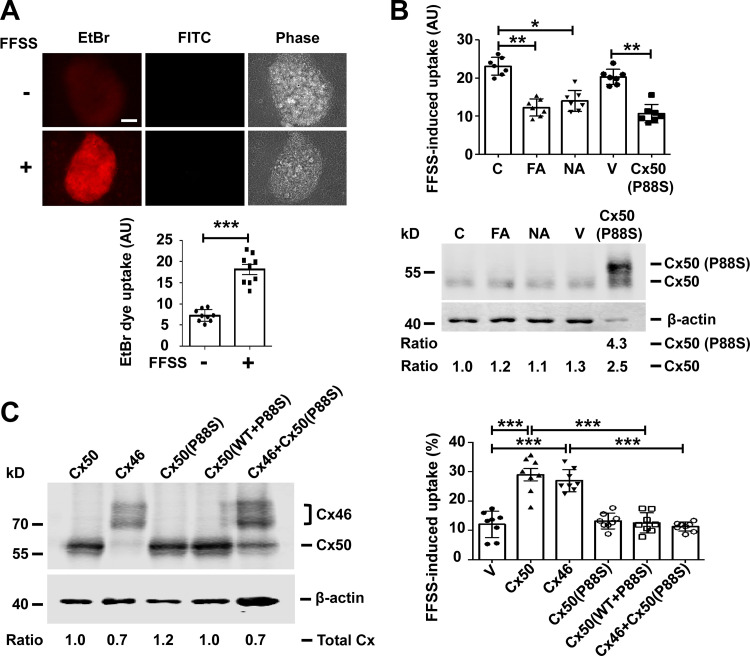
Figure 1.
Cx50 hemichannels are induced open by mechanical stress. (A) Differentiated chick lens primary culture was subjected to FFSS, and EtBr/FITC-dextran dye uptake assay was performed and imaged (upper panel). Scale bar, 50 µm. FITC-dextran (Mr ∼10 kD) is too large to pass through hemichannels in live cells and served as a control for nonspecific permeable, dying cells. The level of EtBr uptake in lentoids was quantified (excluding cells that took up both EtBr and FITC-dextran) using ImageJ (lower panel). Each data point in the graph represents an individual lentoid in one of three independent experiments. (B) Differentiated chick lens primary culture was treated in the absence (C) or presence of 100 µM flufenamic acid (FA) or 100 µM NA for 30 min or infected with high-titer RCAS(A) vehicle (V) or recombinant RCAS(A) retroviruses containing Cx50 mutant P88S. An EtBr uptake assay was performed after 10 min of FFSS at 1 dyn/cm2 (upper panel). Each data point in the graph represents an individual lentoid in one of three independent experiments. Crude cell membrane extracts were immunoblotted with anti-Cx50 loop domain antibody and β-actin antibody (lower panel). The expression of Cx50 in the lentoids was quantified, and the relative ratio of band intensity of Cx50 to housekeeping protein β-actin with the ratio of the control set as 1 is shown underneath the immunoblot. (C) CEF cells were infected with high-titer recombinant RCAS(A) retroviruses containing Cx50, Cx46, or P88S or coinfected with P88S and Cx50 or Cx46. Crude cell membrane extracts were prepared and immunoblotted with anti-FLAG tag and β-actin antibody (left). The expression of retrovirus-induced exogenous connexins in the CEF cells was quantified. The relative ratio of band intensity of total connexins (Cx50 and/or Cx46) to housekeeping protein β-actin with the ratio of the control set as 1 is shown underneath the immunoblot. LY/RD-dextran dye uptake was conducted after FFSS. RD-dextran (RD; Mr ∼10 kD) served as a control for nonspecific permeable, dying cells. The percentage of cells with LY dye uptake (excluding cells that took up both LY and RD-dextran) per image was quantified (right). Each data point in the graph represents an individual quantified image in one of three independent experiments. All data are presented as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.