Figure 1. Cytosolic LPS sensing by the non-canonical inflammasome.
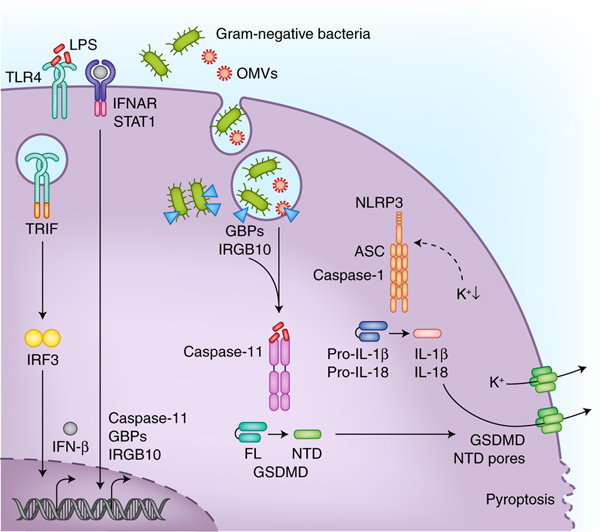
LPS that gains access to the cytosol is sensed by a subfamily of caspases namely caspase-11 in mice and caspase-4 and caspase-5 in humans. The coordinated actions of Guanylate-binding proteins (GBPs) and immunity-related GTPase family member b10 (IRGB10) facilitate the release of LPS from bacteria in the phagosomal vacuole or those that have invaded the cytosol. Outer membrane vesicles (OMVs) secreted by bacteria also enable the cytosolic localization of LPS during infections. The type I interferon signaling initiated downstream of TLR4-TRIF recognition of LPS ensures adequate expression of non-canonical inflammasome components such as caspase-11, GBPs, and IRGB10. Active caspase-11 and caspase-4 cleave gasdermin-D (GSDMD) to liberate its N terminal domain (NTD), which migrates to the plasma membrane forming pores with an inner diameter of about 18 nm. In monocytes, the NLRP3 inflammasome is also activated following GSDMD activation most likely due to the dissipation of intracellular potassium levels through the pores. Accumulation of GSDMD pores on the plasma membrane eventually leads to pyroptotic cell death which occurs in different cell types.
