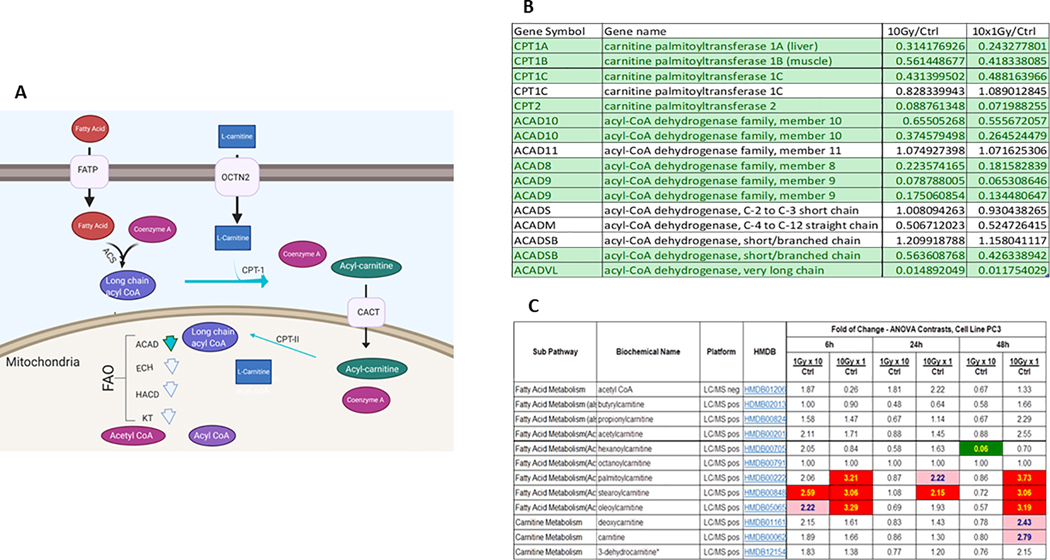
Figure 3: Perturbations in lipid metabolism after SD and MF radiation.
Figure 3A. Schematic representation of long chain fatty acid transport across cell membrane and into the mitochondria for FAO. Fatty acids utilize a protein mediated system to cross the cell membrane, for simplicity this is marked as fatty acid transport protein (FATP). Fatty acids are converted to acyl-CoAs to activate them. To enter the mitochondria, acyl-CoAs must be bound to carnitine. CPT1 converts acyl-Coas to acylcarnitines which allows the fatty acid to enter the inner mitochondrial membrane through CACT. CPTII converts acylcarnitines to acyl-CoAs. The acyl-CoA undergoes FAO. Chain length specific acyl-CoA dehydrogenase (ACAD) perform the initial step of FAO. The following three steps are catalyzed by enoyl-CoA hydratase (ECH), 3-Hydroxyacyl-CoA Dehydrogenases (HACD), and 3-Ketothiolases (KT). This produces one acetyl-CoA, one shortened acyl-CoA, NADH and FADH2, which can be fed into the TCA cycle. The shortened acyl-COA may undergo another round of FAO. Green arrows indicate decreased expression of enzymes in PC3 cells 24h post radiation as indicated in figure 3B.
Figure 3B. Microarray data from human prostate cancer cell PC3 24h after the end of SD or MF radiation exposure. Results are presented as irradiated divided by control. Green indicates that genes were statistically, significantly down regulated by paired T-test. CPT1 is considered the rate limiting enzyme for long chain fatty acid entry into the mitochondria for FAO. CPTII activates acyl-carnitines for FAO. Various members of dehydrogenase family each display specificity for fatty acid chains and are the first step of FAO.
Figure 3C. Metabolic data from PC3 cells 6h, 24h, and 48h after single dose (SD) or multifractionated (MF) radiation. Results indicate significant buildup of specific acylcarnitines at 6 and 48h after single dose(SD) radiation. Asterisk (*) indicates compounds identified based on the mass spectrometry data, but that do not currently have a reference standard to verify the identity.